- Submissions

Full Text
Researches in Arthritis & Bone Study
Osteochondral Lesions of the Talus: A Histopathological Classification
Katharina Kneer1*, Hajo Thermann2 and Irina Berger3
1Department of Neurology and Epileptology, Eberhard Karl University of Tübingen, Germany
2Department for Orthopaedics and Traumatology, ATOS Clinic Heidelberg, Germany
3Department of Pathology Klinikum Kassel, Germany
*Corresponding author:Katharina Kneer, Department of Neurology and Epileptology, Eberhard Karl University of Tübingen, Germany
Submission: April 10, 2023;Published: May 09, 2023
Volume2 Issue1 May , 2023
Abstract
Background: Osteochondral Lesions (OCL) occur due to repeated traumatic injury to bone and cartilage,
triggering pathological changes of subchondral bone. Treatment effects are very variable and often poorly
predictable. To date, there is no histopathological classification available.
Methods: A total of 76 cases with OCL of the ankle (64% male, 26±13 years) were included in the study
and investigated by conventional microscopy and immunohistochemistry (CD56, CD68, CD31).
Result: Four histological types of OCL were identified: Type I aseptic osteonecrosis, Type II Granulation
tissue with neovascularization and moderate inflammatory reaction, Type III Bone regeneration, Type IV
Degenerative changes.
Conclusion: OCL structures are inhomogeneous. We assume that Type I appears as an initial phase of OCL
and can turn into Types II, III or IV, whereas II and III can be seen as a continuous process, Type IV is most
likely not pathologically linked to subtypes II and III. We assume that the type affecting the can influence
the course of healing and treatment outcome and should be considered regarding therapy choice and
initiation. We suggest a pathological investigations following arthroscopy biopsies to investigate and
classify the destruction of cartilage allowing aa evaluation and improvement of treatment success.
Keywords:Osteochondral lesion; Ankle; Classification
Introduction
Osteochondral Lesions (OCL) represent a defect affecting cartilage and subchondral bone of a joint, mainly due to traumatic event. OCL is one of the most common orthopaedic diagnosis in the patient group below the age of 40 mainly affecting sport active patient [1]. Traumatic (or non-traumatic) induced, prolonged and/or repeated damage of the joint surface causes cartilage destruction, bone fragments expulsion to the joint capsule and cyst formation. It is primarily non degenerative disease affect usually weight and movement bearing joints, such as the ankle joint, knee joint, elbow and sometimes also hip and wrist. There is no clinical evidence for genetic predisposure [1-4]. Treatment of OCL is a challenging process despite intensive experimental research and clinical studies [5]. Non-invasive treatment methods based on fixation and immobilisation of the joint are rarely successful. Surgical intervention focuses on excision of lesion, replacement of autogenous bone graft or reconstruction of hyaline cartilage. Indicators for the choice of each treatment remain unclear. Attempts have been made to further cluster OCL in stable and unstable for treatment decision. Stable OCLS heal completely with immobilisation with or without the addition of NSAIDs and do not require surgical intervention. Unstable OCLs require surgical treatment, mainly simple excision of partially detached bone fragments, excision and curettage, curettage and autogenous bone graft, antegrade drilling, retrograde drilling, osteochondral transplantation, autologous chondrocyte implantation or fixation [6,7].
Positive outcomes have been reported with each treatment method available but with the success range varying from 33-90% [7-9]. The outcome of each treatment method is poorly predictable as fare it is still unclear which pathological factors determine the response to each treatment method. For example, so far solely the size of an OCL determines the treatment type. If a lesion is considered to be large, the patient is more likely to receive an allograft transplantation. However, the study of Ramponi et al. [10] failed to show evidence of a significant correlation when comparing OCL lesion size and the outcome after an allograft transplantation [10,11].
Numerous of experimental studies indicate a leading role of functional status of the subchondral bone, such as thickness of the bone trabecule or vascularisation of the subchondrale bone plate in the regeneration of joint cartilage [12,13]. The subchondral bone is able to absorb a main part of the mechanical impact and have strong gross-talk with the joint cartilage using different pathways of the signal transduction [14]. Moreover, it was shown that the subchondral bone and its histomorphology play a crucial role for the functional capacity of joint cartilage [15]. Whereas clinical and radiological criteria for OCL are well defined, systematic histological classifications of OCL are non-existent [5,7]. The present histological definition of OCL is limited to single reports on chondral structural changes [3,5] Degenerative changes of joint cartilage and the progressive loss of chondral substance are therefore mainly described. The radiological classification provided by Berndt and Hardy solely focuses on the characteristics of bone destruction and cannot define ongoing pathological processes in the damaged subchondral bone [16,17]. Treatment options are based on radiological findings [18]. The status of morphological feature of disease will not been considered in the surgical strategy. We suppose that pathological features of OCL are inhomogeneous and can affect not only the progression of lesion but can also provide morphological basis to determine the optimal treatment method. The present study aims to give a systematic histological classification of OCLs, by focusing on ankle joints.
Material and Methods
Tissue samples
Tissue samples were taken from the archive of the Institute of Pathology Kassel from the 01.01.2009 to 31.12.2016. Tissue was taken during arthroscopic intervention or surgical excision of OCLs affecting ankle joints between 2009 and 2016 in the ATOS clinic Heidelberg, Germany. The size of extracted tissue varied from 0.5 to1.5cm. All samples were fixed in 4% formalin in PBS buffer, ETDA decalcified, in paraffin embedded and prepared for diagnostic investigation.
Sample
Inclusion criteria
a. Clinically investigated and MRI imaged Osteochondral
Lesions affecting the ankle joint.
b. Verification of cartilage and subchondral bone in tissue
samples. All tissue samples showing solely cartilage or
subchondral bone were excluded from this study.
c. No previous operative treatment of OCL.
Clinical investigation
The clinical investigation was ensured by the use of arthroscopy and MRI-scanning.
Histology
Tissue sections (4μm thick) were cut from formalin-fixed paraffin-embedded tissue blocks on a microtome (Reichert-Jung), mounted onto adhesive microscope slides and dried overnight at 58 °C. All samples were stained using H&E, PAS and Masson-Goldner staining. In addition, an immunohistochemical investigation was performed.
Immunohistochemistry
All specimens have been investigated by immunohistochemistry. The CD 68 marker, which stains lysosomes add is therefore mainly detected in phagocyting cells, was used to identify osteoclasts and macrophages in the subchondral bone. The CD 56 Marker marks adhesion molecule, which are well known to be expressed in osteoblasts. This marker was used to identify a regeneration of the subchondral bone. CD 31 was used as a marker for detection of regeneration (proliferation) of blood vessels. Tissue sections (4μm thick) were cut from formaline fixed and paraffin embedded tissue blocks on a microtome, mounted onto adhesive microscope slides and dried overnight (58 °C). Immunohistochemical staining (IHC) was carried out by fully automated staining system Ventana Benchmark GX (Roche, Ventana) and the OptiView DAB IHC Detection Kit (Roche, Ventana) at 37 °C according to manufacturer’s recommendations. Following antibodies (all ready-to-use, from Roche, Ventana) were used for IHC: CD68 (clone KP-1, epitope retrieval CC1 for 8 minutes and 24 minutes antibody incubation time). CD56 (clone MRQ-42, epitope retrieval CC1 for 16 minutes and 16 minutes antibody incubation time). CD31 (clone JC70, epitope retrieval CC1 for 8 minutes and 16 minutes antibody incubation time). The stained tissue sections were cover-slipped using Aquatex (Merck), dried for 90 minutes at 55-60 °C and controlled for the quality of staining. An on-slide-positive control was mounted on each slide for quality assurance.
Quantitative evaluation
The quantity of vessels (CD31)/mm2 of subchondral medullary space, quantity of CD68 positive osteoclasts and quantity of CD56 positive osteoblasts/mm2 of bone trabecule were investigated. In addition, we analysed vital trabecular bone volume which was defined as the percentage of the medullary cavity occupied by mineralised trabecular bone. Cellular occupation of trabecular bone lacunes by osteocytes, detectable osteoclasts or osteoblasts, lack of trabecular fragmentation were identified as morphologic criteria for vital bone.
Statistical analysis
Statistical analysis was performed with the R software package. Standard deviations were calculated. Significance analysis was assessed using ANOVA test and one-sided t-test.
Result
76 cases of OCL in ankle joints (64% male, 26±13 years) were included in this study. All probes show cartilagenous degenerative changes, mainly associated with a metaplasia of hyaline cartilage to fibrous cartilage. The chondrocytes were found in an irregular order with an extended loss in the typical column shaped formations. A clear rarification of cell accumulations with a transition to almost acellular chondral sections was observed, not showing any signs of inflammatory infiltrations. Focal transformation of chondrozytes into fibroblasts has been seen (Figure 1 & 2). In contrast to this the subchondral bone findings were highly inhomogenous and varied from necrotic to regenerative and degenerative changes. According to this broad spectrum of the morphological findings, we clustered the morphological figures into four main groups. Table 1 gives a summary for the data in the morphometric analysis.
Figure 1:Representative areas of cartilage and bone affected by OCL
A: H/E stain of aseptic osteonecrotic bone, no inflammatory infiltration, absent osteoblastic/clastic lining of trabeculi,
no neovascularization
B/C: H/E stain proliferation of granulocytic tissue, loose lymphoid tissue infiltration, vascularization
D: CD31 positive testing for neovascularization
E: positive CD68 stain; activation of osteoclasts and Macrophages in subchondral bone
F/H/I: H/E stain; cyst formation, loose connective tissue, no inflammatory infiltration
G: Trichrome GS stain; detection of myxoid degeneration
J: PAS- stain; detection of myxoid degeneration
K: H/E stain; low fibrous tissue density, bone regeneration
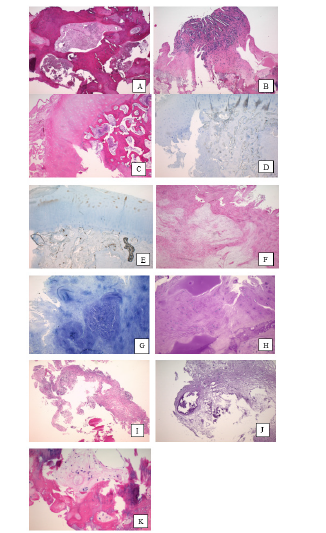
Figure 2:Flowchart of possible development of OCL.
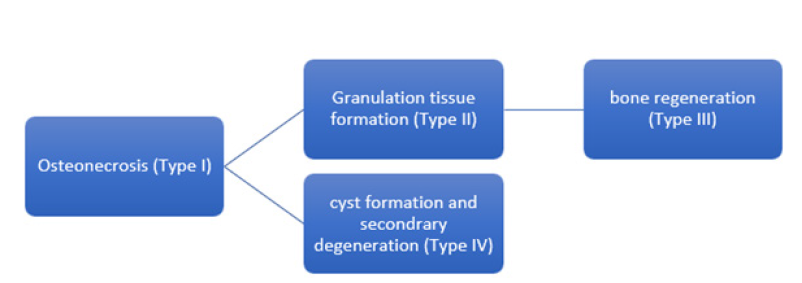
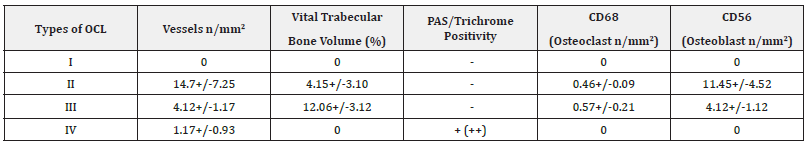
Table 1: Summarized morphometric data of investigated parameters.
Group I: Osteonecrosis (Figure 1A)
Subchondral aseptic osteonecrosis was observed in 9 of 76 cases (11.8%). This was detected as an amorphic acellular material in bone marrow. CD31 staining proofed no vascularisation (0 per mm2). PAS/Trichrome staining remained negative. There was no evidence of osteoclasts or osteoblasts growth.
Group II: Granulation tissue formation with fibrosis (Figure 1B & 1E)
17 of 76 (22.37%) biopsies showed a proliferation of granulation tissue, associated with an increased neovascularisation. CD 31 indicated an activation of vascular proliferation (14.7+/-7.25/ mm2). Alongside the neovascularisation, initial regeneration of the subchondral trabecular bone was found with a vital trabecular bone volume of 4.15+/-3.10%. CD68 staining for osteoclasts as well as CD56 staining for osteoblasts was positive, showing an osteoclast count of 0.46+/- 0.09/mm2 and an osteoblast count of 11.45 +/- 4.52/mm2. No necrosis was observed, and PAS/Trichrome staining remained negative.
Group III: Bone regeneration (Figure 1K)
6 of 76 samples (7.73%) showed an increased subchondral bone volume (12.06+/- 3.12) with a focally well-structured medullary space. CD31 staining was positive, indicating vascular proliferation (4.12+/-1.12/mm2). PAS/ Trichrome staining remained negative. In comparison to Group II, an increasing number of osteoclasts (0.57+/-0.21/mm2) and slightly decreasing number of osteoblasts was found (9.83+/-3.16/mm2). The difference was shown to not be statistically significant (p=1.0)
Group IV: Degenerative changes (Figures 1F-1J)
In 44 of 76 samples (57.7%) myxoid degeneration and subchondral cyst formation were observed. No CD68 and CD56 positive cells were detected along with no signs for bone regeneration. CD31 showed evidence for some revascularisation (1.17+/-0.92 mm2), but also remained negative in 37 cases. PAS/ Trochrome staining was moderately positive. Myxoid matrix showed fragments of joint cartilage and subchondral trabecular bone. The cellularity was represented by metaplastic chondrocytes.
Discussion
Present study showed an inhomogenous pathological process in subchondral part of OCL despite similar clinical feature. We suggest a histological classification of OCL according to the various pathological processes occurring (necrosis, regeneration, degeneration) and define following histological types of OCL: Type I: aseptic necrosis of subchondral bone; Type II: subchondral granulation; Type III: bone regeneration; Type IV: degenerative and cystic changes. Despite the different typology, all our OCL samples showed similar degenerative changes and destruction of the joint cartilage. This is obviously due to pathologically changed microenvironment of the subchondral bone. Starting point in most cases of an Osteochondral Lesion is a traumatic event, initially leading to a local destruction of subchondral bone. Thus, we consider Type I (aseptic necrosis) to be an early stage of OCLs. Our investigations show a complete absence of bone regeneration, inflammatory infiltration and vascularization in this type of OCL. At this stage, the subchondral plate does not provide any morphological basis for chondral regeneration. OCL Type II and Type III represent subchondral regeneration. In both subtypes vascular proliferation and new bone formation can be identified. Type II shows a significant (p<0.05) higher vascular proliferation and the increasing number of new vessels. Type III shows a reduction in vessel density with a differentiation of vessels accompanying an incline in bone density. We observed a progressive increase expression of osteoclasts and osteoblasts as a sign of an activated bone turn over and bone regeneration in this type of OCL. We assume that Type II and Type III of OCL represent different stages of the bone regeneration process (Figure 2). High vascularization of subchrondral membrane and bone synthesis provide a positive morphological basis/microenvironment for cartilage regeneration. Several clinical studies report a poor outcome of the chondroplasty or of the osteochondral transplantation in OCL due to the avascularity of the subchondral bone and suggest a microfracture procedure as a possible way to improve a subchondral vascularisation and its presence is linked to less cartilage damage [19-22]. We assume a better outcome of transplantation methods, such as chondral cell and matrix transplantation using for treatment of Type II and Type III OCL. Moreover, we suspect a possibility of spontaneous regeneration of these types of OCL. A complete surgical excision of the bone regenerate could be contraindicative from a morphological point of view; hence a starting patient-own regenerate would be damaged.
Type IV shows in contrast to other types of OCL primarily degenerative changes. No signs of bone regeneration, no vascularisation of the subchondral area is found. Partially, a tendency for a cystic transformation can be seen. We assume that this subtype cannot be linked to Type II and III, neither chronologically, nor pathologically. It reflects an inert pathological protracted process. From a morphological point of view, there is no possibility of an adequate spontaneous chondral and osseous regeneration. Therefore, regarding Type IV (degenerative changes) and Type I (necrotic changes), cartilage and matrix transplantation would remain unsuccessful due to the environmental conditions in which the transplant is embedded. Based on results of the present study we suggest following scenario of OCL development (Figure 2). Traumatic or non-traumatic impact leads to aseptic necrosis of subchondral bone (OCL Type I) and therefore leads to essential changes of environment of the joint surface resulting in destruction of joint cartilage. During further progression of disease, a resorption of the aseptic osteonecrosis is induced. Depending on the effectiveness of this process, either granulation tissue formation (Type II) or bone regeneration (Type III) occurs. In case of an insufficient regeneration, degenerative processes and cysts regression dominate in pathological feature (Type IV). Type IV could represent a non-recurrent pathological process, whereas Type II and III could represent different stages of a physiological regenerative process (Figure 2). We suppose that a success of OCL treatment can be strongly affected by knowledge about status of subchondral bone defined as one histological Type of OCL. We suggest a consideration of histological OCL Type for the treatment strategy.
Based on our results, we assume that the poor predictability of each OCL treatment method available is caused by variable pathological processes in OCL and thus these processes have to be considered when choosing a treatment option. Further, these in homogenic histopathological findings underline current clinical studies, suggesting that neither size nor location of the OCL seem to have a positive outcome after surgical treatment [23]. Moreover, systematic studies are needed to define, which pathologic processes influence development of each Type of OCL. Possible pathways for linkage of Type I of OCL into Types II and III should be proved. The study of Hoemann et al. [3] suggests a positive impact of cartilage biopsy for the analysis of cartilage regeneration [3]. Solely an analysis of the cartilage is not sufficient enough to determine the prognosis of an OCL. The regeneration of joint cartilage is strongly influenced by the tissue environment of the subchondral mass. An adequate analysis of regenerative capacity of joint cartilage can be only made when therefore the subchondral area is being drawn into the analysis too. Further, not only a closer analysis of the microenvironment, but also the taking a closer look at the macroenvironment of the affected bone, such as ligament malalignments, should be taken into consideration to further aid healing processes [24,25]. We hypothesize that taking the histopathological picture of an OCL into account when choosing the treatment, can have an impact on the course of healing. The overwhelming importance of the pathological conditions of the subchondral bone plate has to be addressed with new diagnostic and treatment concept. Cartilage reconstruction by all means (ACT, AMIC et.) is not the solution to resolve this complex problem. To clarify this, interdisciplinary long-term studies are needed, maybe pre- and post-operative biopsies of OCLs (in cases of failure) should be taken and the presenting OCL- type should be considered when planning the choice of therapy method. New treatment concepts should include improvements of the subchondral bone plate regeneration.
Conclusion
OCL morphology shows an inhomogeneous structure of the subchondral bone. Four Types of OCL can be defined: Type I: necrosis; Type II Granulation; Type III bone regeneration; Type IV degeneration. Due to detectable regenerative processes in Type II and Type III of OCL we suppose a good healing potential and a good outcome of the transplantative options of surgical treatment in these Types of OCL. We hypothesize that knowledge about pre-surgical status of subchondral bone defined by histological classification of OCL can be helpful for choice of treatment method and therefore can help to optimize treatment outcome.
Acknowledgement
We acknowledge support by Open Access Publishing Fund of the University of Tübingen.
References
- Schewe B, Fritz J, Weis K (2008) Cartilage injuries in the knee joint orthopedics and trauma surgery. University of Münster, Germany.
- Canale ST, Belding RH (1980) Osteochondral lesions of the talus. J Bone Joint Surg Am 62(1): 97-102.
- Hoemann C, Tran KN, Chevrier A, Chen G, Lascau CV, et al. (2015) Chondroinduction is the main cartilage repair response to microfracture and microfracture with BST-CarGel: Results as shown by ICRS-II histological scoring and a novel zonal collagen type scoring method of human clinical biopsy specimens. Am J Sports Med 43(10): 2469-2480.
- Körner D, Kohler P, Schröter S, Naumann A, Walther M, et al. (2018) Pain in osteochondral lesions of the ankle - An investigation based on data from the German cartilage registry (Cartilage Register DGOU). Journal of Orthopedics and Trauma Surgery 156(2): 160-167.
- Shimozono Y, Hurley E, Yasui Y, Deyer T, Kennedy J (2018) The presence and degree of bone marrow edema influence midterm clinical outcomes after microfracture for osteochondral lesions of the talus. The American Journal of Sports Medicine 46(10): 2503-2508.
- Zanon G, Di Vico G, Marullo M (2014) Osteochondritis dissecans of the talus. Joints, pp. 115-123.
- Zengerink M, Struijs PAA, Tol JL, Dijk CN (2010) Treatment of osteochondral lesions of the talus: A systematic review. Knee Surg Sports Traumatol Arthrosc 18(2): 238-246.
- Jackson A, Drayer N, Samona J, Dukes C, Chen C, et al. (2019) Osteochondral allograft transplantation surgery for osteochondral lesions of the talus in athletes. The Journal of Foot and Ankle Surgery 58(4): 623-627.
- Takahara M, Mura N, Sasaki J, Harada M, Ogino T (2008) Classification, treatment, and outcome of osteochondritis dissecans of the humeral capitellum. Surgical technique. J Bone Joint Surg Am 90(2): 47-62.
- Ramponi L, Yasui Y, Murawski C, Ferkel R, Di Giovanni C, et al. (2016) Lesion size is a predictor of clinical outcomes after bone marrow stimulation for osteochondral lesions of the talus: A Systematic Review. The American Journal of Sports Medicine 45(7): 1698-1705.
- Ferrari M, Sanchez G, Chang A, Sanchez A, Ellera GJ, et al. (2017) Osteochondral allograft transplantation for treatment of focal patellar osteochondral lesion. Arthroscopy Techniques 6(4): e907-e912.
- Stewart H, Kawcak C (2018) The importance of subchondral bone in the pathophysiology of osteoarthritis. Frontiers in Veterinary Science 5: 178.
- Yasui Y, Ramponi L, Seow D, Hurley E, Miyamoto W, et al. (2017) Systematic review of bone marrow stimulation for osteochondral lesion of talus - evaluation for level and quality of clinical studies. World Journal of Orthopedics 8(12): 956-963.
- Shimozono Y, Yasui Y, Ross A, Kennedy J (2017) Osteochondral lesions of the talus in the athlete: up to date review. Current Reviews in Musculoskeletal Medicine 10(1): 131-140.
- Fell N, Lawless B, Cox S, Cooke M, Eisenstein N, et al. (2019) The role of subchondral bone, and its histomorphology, on the dynamic viscoelasticity of cartilage, bone and osteochondral cores. Osteoarthritis and Cartilage 27(3): 535-543.
- Hepple S, Winson IG, Glew D (1999) Osteochondral lesions of the talus: A revised classification. Foot Ankle Int 20(12): 789-793.
- Petrie PW (1977) Aetiology of osteochondritis dissecans. Failure to establish a familial background. J Bone Joint Surg Br 59(3): 366-367.
- Santrock R, Buchanan M, Lee T, Berlet G (2003) Osteochondral lesions of the talus. Foot and Ankle Clinics 8(1): 73-90.
- Madry H, Van Dijk C, Mueller GM (2010) The basic science of the subchondral bone. Knee Surgery, Sports Traumatology, Arthroscopy 18(4): 419-433.
- Santo V, Gomes M, Mano J, Reis R (2013) Controlled release strategies for bone, cartilage, and osteochondral engineering-Part I: Recapitulation of native tissue healing and variables for the design of delivery systems. Tissue Engineering Part B: Reviews 19(4): 308-326.
- Santo V, Gomes M, Mano J, Reis R (2013) Controlled release strategies for bone, cartilage, and osteochondral engineering-Part II: Challenges on the evolution from single to multiple bioactive factor delivery. Tissue Engineering Part B: Reviews 19(4): 327-352.
- Zarka M, Hay E, Ostertag A, Marty C, Chappard C, et al. (2019) Microcracks in subchondral bone plate is linked to less cartilage damage. Bone 123: 1-7.
- Seo SG, Kim JS, Seo DK, Kim YK, Lee SH, et al. (2018) Osteochondral lesions of the talus. Acta Orthopaedica 89(4): 462-467.
- Batista J, Joannas G, Casola L, Logioco L, Arrondo G (2021) New classification of osteochondral lesions of the talus in adults. Journal of the Foot & Ankle 15(1): 77-82.
- Krause F, Anwander H (2022) Osteochondral lesion of the talus: Still a problem? EFORT Open Reviews 7(6): 337-343.
© 2023 Katharina Kneer. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






