- Submissions
Full Text
Researches in Arthritis & Bone Study
Ceramics in Arthroplasty, Arthritis and Orthopaedics
Prakash L*
Director and Chief of Orthopaedics, Institute for Special Orthopaedics, India
*Corresponding author: L Prakash, Director and Chief of Orthopaedics, Institute for Special Orthopaedics, Chennai, India
Submission: February 23, 2018; Published: March 23, 2018
Volume 1 Issue 1 March 2018
Introduction
Ceramics are non-metallic inorganic materials and vary in composition. They are made by mixing the fine powders of the ingredient material with water and adhesive binder. This is then squeezed into a mould to obtain the desired shape, air dried to dry, and the binder is then burned out by thermal treatment. Firing or sintering at this stage at a high temperature (over 1000 degrees Celsius) makes the residual material extremely dense. Modern ceramics do not use either water or binding agents to bind or compress the ceramic shapes, and use an Isostatic Pressing Technology, to fuse the ceramic granules under very high pressure to form a non hygroscopic and non water dependant unit, of such high strength, that after high temperature fusion, the resultant component can even be machined on a conventional Cnc machine like metals. The final microscopic structure of the resultant ceramic is greatly dependent on the thermal process used, the highest temperature reached, and the duration of furnace heat treatment.
Five types of ceramics are used in arthroplasty:
A. Glass,
B. Plasma-sprayed polycrystalline ceramic,
C. Vitrified ceramic,
D. Solid state sintered ceramic
E. Polycrystalline glass-ceramic
Other factors determining the mechanical and biological properties are the purity of the powder, the size and distribution of the grains and the porosity. Ceramics used in orthopedic surgery are classified as bioactive or inert according to the tissue response when implanted in an osseous environment. The bioactivity of a material can be defined as its ability to bond biologically to bone. An inert ceramic merely elicits a minor fibrous reaction. In clinical practice, inert fully-dense ceramics are used as bearings in total joint replacements because of their exceptional resistance to wear and bioactive ceramics are used as coatings to enhance the fixation of the femoral stems or acetabular shells.
Sliding ceramics
The most widely used bearing combination in total joint replacements is metal-on-polyethylene. The long-term survival of the artificial joint however, is dependent on the wear of its components. It is the poly wear, which ultimately leads to osteolysis around the implant leading to an inflammatory response induced by wear debris occurring from both the articulating and nonarticulating surfaces. Pierre Boutin first introduced ceramics in orthopaedics in the early 1970s in order to tackle complications related to polyethylene wear. Ceramics are mainly used in total hip arthroplasty as femoral heads articulating against polyethylene and as cups in the alumina-on-alumina combination. Because of their relatively brittle nature, fracture of ceramic femoral heads has been, along with cost, the main limitation to their expanded use world-wide. The risk of fracture, however, has been virtually eliminated because of a great improvement in the manufacturing process with increased purity and density, increase in the size and distribution of the grains and better quality control. This has mostly been achieved by Isostatic pressing technology and enhanced purification methods of the ceramic clays. Accurate fixation of the ceramic ball to the femoral stem through a well-designed Morse taper avoids undesirable stresses in the head and improved surgical techniques augment the longevity of the implant. Currently most ceramic femoral heads have a 12mm by 14mm Morse Taper, making them compatible with most metal femoral stems. The fracture rate of ceramic heads has been evaluated as 0.02% for alumina heads and 0.03% for those of zirconia indicating that fracture of the ceramic head is no longer an important factor. Cost however still is a limiting factor as ceramic heads are six to ten times as expensive as metal heads.
Alumina ceramic
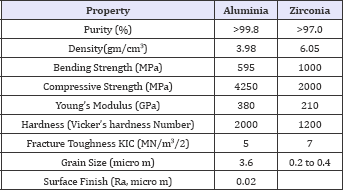
Dense alumina of surgical grade is obtained by sintering alumina powder at temperatures between 1600 and 1800 °C. The resultant material is in its highest state of oxidation, allowing thermodynamic stability, chemical inertness and excellent resistance to corrosion. Improvement in the manufacturing process has lowered the size and distribution of the grains, which are major factors in avoiding cracks and fracture. Alumina is a brittle material with excellent compression strength but the bending strength is limited. The Young's modulus is 300 times greater than that of can cellous bone and 190 times higher than polymethylmethacrylate (PMMA). Alumina has been a standardized material since 1984 (International Standard Organisation, ISO 6474). The tribological properties of alumina ceramic against itself are outstanding with a linear wear rate 4000 times lower than that of metal-on- polyethylene. The excellent frictional characteristics are due in part to a high wet ability because of the hydrophilic surface and fluid film lubrication which minimizes adhesive wear. These properties, demonstrated both in vitro and on analysis of retrieved implants are responsible for the limited amount of wear particles produced and the subsequent moderate biological reaction to ceramic wear debris. The clearance between the two components in the case of the alumina-on-alumina combination should be around 50 nanometers to avoid Hertz stresses at the surface of the alumina which may result in detachment of grains and third- body wear. This Hertz stress is a geometrically progressive wear, as the particles produced exponentially Wroblewski, Siney and Fleming recently described the results of 22mm alumina femoral heads articulating against cross-linked polyethylene in a ten-year follow-up. A running-in rate of penetration was noted which then decreased to 0.022mm/year after the first 18 months. It is difficult, however, to conclude from this study whether the alumina head, the small diameter of the femoral head, or the cross-linked nature of the polyethylene cups was responsible for the low rate of wear observed. Comparative physical properties of alumina and zirconia ceramics of surgical grade Zirconia ceramic was introduced in the manufacture of femoral heads for total hip replacements because of its higher strength and toughness which would reduce the risk of fracture. Pure zirconia is an unstable material showing three different crystalline phases: monoclinic, tetragonal and cubic. The phase changes result in a large variation in volume and significantly decrease the mechanical properties of the material due to the production of cracks. Stabilization of zirconia by adding oxides to maintain the tetragonal phase has therefore been undertaken. Yttrium-stabilized tetragonal polycrystalline zirconia (Y-TZP) has a fine grain size and offers the best mechanical properties (Table 1). This material was standardized in 1997 (International Standard Organisation, ISO 13356). Zirconia femoral heads should articulate only against polyethylene sockets since both zirconia against alumina and zirconia against zirconia-20 have been shown to produce catastrophic rates of wear in vitro. Zirconia-on-polyethylene has demonstrated similar rates of wear as alumina-on-polyethylene in vitro, but in vivo the results have not been so favorable. Allain et al. recently described a consecutive series of 78 hips using a zirconia femoral head and a polyethylene cup. Complete radiolucent lines were observed around the cup in 23% of the hips and 17% of the femoral implants had radiolucency greater than 1mm. Survival at eight years was 63%. These worrying results were confirmed by Hernigou and Babrami in a study comparing wear of the cup and osteolysis in 40 hips over a period of ten years. Two comparable groups of 20 hips each had either a 32 mm alumina or a 28mm zirconia femoral head. During the first five years, the zirconia group had a lower rate of wear of 0.04mm/year compared with 0.08mm/ year, and osteolysis on the calcar measured in square millimetres was similar in both groups. Between five and ten years, however, the rate of wear increased dramatically in the zirconia group to 0.15mm/ year at ten years as opposed to 0.07mm/year in the alumina group. Osteolysis of the calcar was significantly greater in the zirconia group at 135mm2 compared with 65mm. These results are of concern. The long-term performance of zirconia ceramic may well be altered by degradation in vivo with transformation of the material into its monoclinic unstable phase. Another explanation was suggested by Lu and Mc Kellop who measured the frictional heating of polyethylene cups in a hip joint simulator. The steady- state temperature of the polyethylene reached 99 °C with heads of zirconia ceramic compared with 45 °C with alumina prostheses. This may account for the long-term wear because of the consequent structural changes and may also produce precipitation of lubricant proteins. Better results are apparent in current clinical trials.
Table 1
Mixed-oxide ceramics
A new class of materials has been developed recently to combine the tribological properties of alumina and the mechanical characteristics of yitrium stabilized zirconia. These mixed-oxide ceramics containing 40% to 80% zirconia have shown rates of wear in vitro comparable to those of alumina ceramic. Preliminary results in hip joint simulators have been promising, but further investigations are needed to assess their long-term performance.
Bioactive ceramics
These are osteo-conductive acting as a scaffold to enhance bone formation on their surface and are used either as a coating on various substrates or to fill bone defects. An osteo-conductive material can only elicit bone formation in an osseous environment, whereas an osteo-inductive substance can promote bone formation even in an extra osseous situation.
Calcium phosphate ceramics
Two bio-ceramics belonging to the calcium phosphate family have had extensive evaluation as orthopedic implants, namely HA and tricalcium phosphate (TCP). Stochiometric synthetic HA (Ca10 (PO4)6(OH)2), with a calcium-to-phosphate atomic ratio of 1.67, was introduced as a bone-graft substitute because its formula is similar to that of the inorganic mineral phase of bone. Biological HA, however, is Ca deficient and a carbonated apatite. The bonding mechanism of HA to bone, although not completely understood, seems to be due to the attachment at the surface of the HA of osteogenically- competent cells which differentiate into osteo-blasts. A cellular bone matrix is then formed at the surface of the HA. An amorphous area is present between the surface and the bone tissue containing thin apatite crystals. As maturation occurs, this bonding zone shrinks and HA becomes attached to bone through a thin epitaxial layer, resulting in a strong interface with no layer of fibrous tissue interposed between the bone and HA. Bone formation grows from the surface of the HA towards the centre of the pores. HA coating is widely used on femoral prostheses and on sockets as a means of fixation in order to avoid complications related to the use of PMMA. It is usually applied by plasma spray. An American multicentre study has reported excellent results, with a rate of femoral revision of 0.3% at a mean follow-up of 8.1 years, with one case of loosening out of 324 implants. However it has not yet been clearly shown that HA offers improved fixation when compared with bone cement. The thickness of the coating the chemical composition of the material and the roughness and nature of the metal substrate seem to be key factors in ensuring good results. The main disadvantages which have limited the clinical application of HA as a bone-graft substitute are related to the brittle nature and poor tensile strength of the material. Consequently, information on the clinical use of ceramic bone-graft substitutes is scarce. TCP has been evaluated in spinal fusion with results comparable to those with autogenous bone.
Bioactive glasses
Bioactive glasses were first developed by Hench and Wilson and have a vitreous structure. They bond chemically to bone. The model in this class of materials is Bio-glass 45S5 of which the composition in weight % is: 45% SiO2, 24.5% CaO, 6% P2O5 and 24.5% Na2O.
The bonding mechanism of silicate bioactive glasses to bone has been attributed to a series of surface reactions ultimately leading to the formation of a hydroxy-carbonate apatite layer at the glass surface. Greater production of bone has been demonstrated with Bio-glass 45S5 when compared with HA, but due to its poor mechanical properties this material has not been used in load- bearing applications. Recently, in order to improve the reactivity of the material, sol-gel processed glasses, hydrolyzed at ambient temperatures, have been developed to obtain bioactive gel glasses in the SiO2-CaO-P2O5 system, with an initial high specific surface area. These materials have similar osteo-conductive properties to melt-derived glasses, but have an improved degradability. The low temperatures used to produce sol-gel glasses allow them to be used as a coating on alumina substrates. When implanted in an animal model, sol-gel glass-coated alumina has demonstrated the ability to form an interface mainly composed of newly-formed bone by 24 weeks. In this class of material, apatite wollastonite (CaOSiO2) glass ceramic developed by Kokubo et al. has osteo-conductive properties similar to Bio-glass 45S5 but increased mechanical strength. It has been used as a spacer at the iliac crest, for vertebral prostheses and as a shelf in procedures about the shoulder with favorable results.
Bioactive bone cements
Bioactive bone cements have been explored as an alternative to PMMA in order to avoid complications related to PMMA debris and to enhance fixation of the prosthesis. These materials have undergone extensive basic research. They include calcium-phosphate based bone cement and glass-ceramic bone cement. A strong cement- bone interface is obtained by the formation of HA at the surface of the cement. Moreover, calcium phosphate cements are restorable and are progressively replaced by newly-formed bone. Ceramic coatings provide an attractive alternative for biological fixation. In the near future, ceramic substitutes for bone grafts will probably be used in association with osteo-inductive materials such as bone morphogenetic proteins or mesenchymal stem cells to accelerate bone formation further (Figure 1-5).
Figure 1:The ceramics used in hips are no different used in our toilets and wash basins. The matrial and the technology are same.

Figure 2: Currently the most popular use of ceramics is as a bearing with HDPE.

Figure 3: The alumina and zirconia ceramics are not too different in look and feel on the operating table.

Figure 4: A zirconia ceramic head with a hydroxyappatite coated stem.

Figure 5: A Hydroxyappatite coated stem.

 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






