- Submissions
Full Text
Researches in Arthritis & Bone Study
When Two Diseases Cross their Paths: The Diagnostic Challenge of Rheumatoid Arthritis in Sickle Cell Disease Patients
Isabel M McFarlane*
Department of Internal Medicine, State University of New York, USA
*Corresponding author: Isabel M McFarlane, Clinical Assistant Professor of Medicine, Associate Program Director Residency Program, Department of Internal Medicine, Division of Rheumatology, State University of New York Downstate Medical Center, New York, USA
Submission: October 18, 2017; Published: March 12, 2018
Volume 1 Issue 1 March 2018
Introduction
When evaluating joint complaints in adult sickle cell disease (SCD) patients, a number of sickle cell-based entities come to mind such as avascular necrosis, osteomyelitis, bone infarcts, and septic arthritis. However, another important cause of joint disease is to be considered, rheumatoid arthritis (RA), this diagnosis is generally overlooked and rarely considered. Anecdotal reports highlighted the occurrence of RA in SCD presenting as diagnostic challenges for cases with chronic inflammatory arthritis, joint effusions, erosive arthritis and non-gouty arthritis [1-3]. SCD itself causes chronic musculoskeletal complaints [4] that may delay or even obscure the diagnosis of RA. Therefore, high clinical suspicion along with laboratory and imaging studies are necessary for the identification of RA in SCD population. Contrary to the previous reports indicating rare coexistence; a study conducted by our group among inner city SCD patients suggest that the prevalence of RA coexisting with SCD is quite similar to the prevalence of RA reported in the general population [5].
RA is a chronic systemic inflammatory disease characterized by inflammation and synovitis leading to damage of cartilage and juxta-articular bone destruction [6]. Environmental factors (smoking and infection), as well as genetic predisposition, are known to play a role in the development of RA. Among Caucasian patients, the human leukocyte antigen-DRB1 (HLA-DRB1) alleles containing the shared epitope, are markers of disease risk and severity. However, HLADRB1 has been found in only one third of African-Americans (AA) RA patients [7]. Single nucleotide polymorphisms genome studies on AA (healthy and with RA) and Caucasians, revealed differences in the allele frequencies in the Tumor Necrosis Factor (TNF) receptor genes suggesting a higher likelihood to develop RA and increased disease severity among AA patients [8]. Higher expression of Interferon y receptor 1 and 2 genes in blood cells of AA with RA also correlated with increased radiographic severity, implying a potential pathogenic role of IFN-y in terms of susceptibility and aggressiveness of disease [9]. AA with RA sustain early damage in the course of the disease and undergo a radiographic progression similar to that seen in other ethnic groups [10].
In SCD, the red blood cells (RBC) dehydrate causing increased viscosity of the cytosol; RBC become unable to maintain their flexibility and shape and acquire the typical sickle shape [11]. RBC membrane in SCD have a tendency to adhere to the endothelium [12,13]. Vaso-occlusive crises (VOC) results in blockage of the blood flow by the sickled red cells which lead to acute chest syndrome, ischemia, stroke, infarcts, pain, bone marrow degeneration and bony infarcts. The Centers for Disease Control and Prevention estimate that about 100,000 Americans are affected by SCD with a life expeRABSncy of 43 and 41 years of age, for women and men respectively [14,15].
Hydroxyurea and advanced medical care has led to the improved survival observed among SCD patients. This prolonged survival however ushers in, a chronic inflammatory disorder, rheumatoid arthritis (RA), which traditionally presents at a relatively older age. Our study revealed that the prevalence of SCD coexisting with RA was 0.94%, which is similar to the prevalence of RA among the general population. Our patients, mostly from Afro-Caribbean descent, with SCD-RA were compared to age and sex matched patients with SCD and RA. The SCD-RA patients had significantly lower hemoglobin and tended to have a lower BMI, increased periarticular osteopenia, erosive arthritis, prolonged morning stiffness, increased number of hospitalizations and longer hospital stay [5].
In our study, SCD-RA patients were also older than the SCD-only population but younger than RA-only patients, indicating earlier occurrence of RA among SCD patients where SCD-RA patients, had been diagnosed with RA about 5 years earlier than RA only patients [5]. The diagnosis of RA at a younger age in SCD patients likely resulted from the frequent medical encounters, although the role of inflammatory markers released during VOC might explain earlier RA presentation among SCD patients. Whether SCD coexisting with RA is merely coincidental or related to shared mechanisms of inflammation will be discussed in the next section.
During VOC, the sickled RBC membrane acquires an enhanced ability to adhere to the endothelium by exposing adhesion molecules [12,13]. Endothelial cell membrane receptors interact with a number of adhesion molecules presented by the sickled RBC [16]. Adhesion molecules promote leukocyte adhesion which leads to luminal narrowing, red blood cell entrapment, vaso-occlusion and inflammation. The interactions between the endothelium and adhesion molecules lead to activation of Nuclear Factor-Kappa peta (NF-kβ) which in turn drives the production of reactive oxygen species (ROS). Inflammation leads to recruitment of leukocytes and platelets, which in turn release proinflammatory cytokines, such as Placental Growth Factor, Tumor Necrosis Factor-a, Interleukinl (IL- 1), and Interleukin-6 (IL-6) among others [16,17]. IL-1p, IL-8, IL-6, IFN-y, hs-CRP, sVCAM-1 and Tissue Factor were also found to be elevated in SCD patients when compared to normal controls, even when not in VOC [18-20]. Repeated VOC in SCD allows for chronic inflammation. Additionally, intravascular hemolysis contributes to inflammation of the endothelium and sub-endothelium smooth muscle layers leading to oxidative stress [20].
Elevated heme levels in circulation can activate innate immunity via Toll-like Receptor-4, which in turn can trigger VOC; damage-associated molecular patterns (DAMP) seem to play important role in pathogenesis of VOC in SCD as well [21]. SCD also creates a procoagulant state via exposed phosphatidylserine on the RBC membrane [19,22]. P-selectin [23] and E-selectin mediated neutrophil adhesion to the endothelium seems to play a pivotal role in VOC. Recruited neutrophils lead to further entrapment of RBC and to a lesser extent platelets, allowing cellular aggregates to form which contributes to inflammation via the production of ROS [24-29].
In RA, the humoral adaptive and innate immune systems are involved. Neoepitopes presented by the inflamed synovial membrane attract plasmacytoid dendritic cells to mobilize into the altered synovium. Macrophages are activated via T helper 1 cytokines like interferon-y, IL-12, 15, 18, 23. Th17 cells also produce IL-17, 21, 22 and TNF-α. IL-17 and TNF-α which promote activation of fibroblasts and chondrocytes. Synovial B cells within the synovial follicles produce not only autoantibodies but also IL-6, TNF-α, and Lymphotoxin-fê that play a pathogenic role RA [6]. TNF-α activate metalloproteinases leading to cartilage destruction and osteoclast activation which contributes to joint damage and erosions [30]. Leukocyte adhesion plays a key role in the infiltration of immune cells into inflamed synovium, facilitating the development and perpetuation of chronic inflammation in RA [6,31,32]. Oxidative damage of the cartilage, extracellular collagen and DNA have been demonstrated along with upregulation of NO in RA synovium which is able to induce TNF-α production, factors that contribute to the inflammatory process in RA [33,34].
Therefore, there is an overlap between SCD and RA pathophysiology that might help explain our observations. We can postulate that elevations in TNF-α, IL-1 and IL-6 and other pro- inflammatory cytokines during SCD VOC may trigger synovitis in RA susceptible SCD patients; in addition, ROS generated during VOC can contribute to the inflammatory process of SCD-RA, leading to a more severe disease course [35]. Ischemic changes in SCD synovium could lead to the release of synovial antigen into the circulation thus inducing autoantibody formation. This theory could explain why SCD patients were diagnosed with RA at a younger age (5 years earlier), tended to be seropositive for RF and anti-CCP than RA only patients and have a tendency to more erosive disease [5]. Mechanistic questions remain to be answered in future research.
From the previous discussion, reaching a diagnosis of RA in a SCD patient requires a thorough history, physical exam, laboratory tests, imaging studies and RA criteria fulfilment [36]. However, the clinician faces a new challenge as the choice of medications becomes controversial. Corticosteroids, first line agents in inflammatory disorders have been reported to trigger sickle cell crisis [37-42]. Therefore, based on the literature steroids should be used in short courses and aggressive hydration should be provided as in an effort to abort an upcoming VOC. Disease modifying anti-rheumatic drugs (DMARDS) are standard of care for RA patients. Hydrochloroquine along with hydroxyurea should be considered when appropriate follow-up of liver function tests and cell blood counts are part of the management plan [43].
There is no literature on the concomitant use of Sulfasalazine and Hydroxyurea among patients with SCD and RA [43]. Methotrexate (MTX) was used in SCD patients in a pilot study conducted by Brandalise et al [44], to evaluate the impact of MTX on SCD VOC. There was no reduction in the frequency of VOC. However, avascular necrosis pain was reduced which impacted the physical function of the patients and suggested some benefit with the combination of MTX and Hydroxyurea. A drop in the platelet counts was observed as well as some impairment in the reticulocyte response. Therefore, study concluded that if MTX is to be used along Hydroxyurea, blood cell counts, reticulocytes and liver function tests should be monitored closely. Leflunomide administration would be often limited by the abnormal liver tests associated with SCD and Azathioprine by the increased risk of myelosuppression.
TNF-α blockers' efficacy record in RA and their effect on the expression of adhesion molecules in the vascular endothelium [45] are an attractive choice of treatment for SCD with RA although literature reports on the use of anti-TNF-α is scanty and no conclusions could be drawn [35]. Biologics targeting the adhesion molecules located in endothelial cells and platelet adhesion have been postulated to impact the number of VOC among SCD. Ataga et al. [23] studied the P-selectin inhibitor in a double blind, randomized placebo-controlled fashion in almost 200 SCD patients.
The annual rate of hospitalization, number of VOC, complicated and uncomplicated events were recorded. From the pathophysiology of RA and SCD previously discussed, a reduction in the number of VOC among SCD should have a beneficial effect among SCD RA patient. Therefore, the SUSTAIN study results open the possibility of a novel approach for the management of SCD-RA patients. We could never stress enough the importance of vaccination among SCD population as well as appropriate screening for tuberculosis and glucose-6-phosphate dehydrogenase testing when anti-TNF and hydroxychloroquine respectively are being considered. As recommended by American College of Rheumatology, patients started on DMARDs should have blood cell counts and chemistries including liver function as per recommended intervals [46].
Conclusion
In conclusion, the next time you evaluate a SCD patient who presents with chronic synovitis, strongly consider the possibility of coexistence of RA and SCD; keeping in mind that the musculoskeletal symptoms in SCD can masquerade and delay the diagnosis of RA in this patient population. It is possible that the underlying inflammatory mechanisms present in SCD and RA may worsen the clinical manifestations of each disease, therefore affecting the patient s prognosis. Management of SCD and RA cases could also be complicated by occurrence of VOC, hemolysis, asplenia and adverse reactions associated with currently available therapies. Until future longitudinal studies to evaluate the clinical course, disease activity, radiological findings and impact on the quality of life, clinicians should be aware of the co-existence of these 2 illnesses and initiate work up and therapy based on the currently available data (Figure 1).
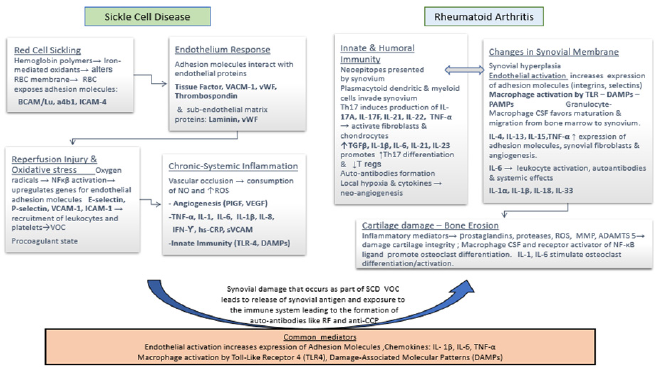
Figure 1: Pathogenesis of Sickle Cell Disease and Rheumatoid Arthritis: Postulated Mechanism and Common Mediators Involved. ADAMTS5 a disintegrin and metalloproteinase with thrombo spondin motifs 5, a4b integrin on platelet surface that mediates adhesion to collagen, BCAM/Lu Basal Cell Adhesion Molecule Lutheran blood group, CSF colony stimulating factor, DAMPs Damage-associated molecular patterns, hs-CRP high sensitivity C reactive protein, ICAM-1 Intercellular adhesion molecule 1, IL interleukin, INF interferon, NF-κβ Nuclear Factor-Kappa Beta, PlGF platelet growth factor RBC red cell membrane, RF Rheumatoid Factor, ROS reactive oxygen species, sVCAM serum vascular adhesion molecule, TL-4 toll like receptor 4, TGF-β Tissue growth factor β, TNF-α tumor necrosis factor β, T regs Regulatory T cells, MMP matrix metalloproteinases, VACM-1 vascular cell adhesion molecule, VEGF vascular endothelium growth factor, VOC vaso-occlusive crises, vWF Von Willebrand Factor.
References
- Coodley ELK, Sickle MJ (1949) Cell disease simulating advanced rheumatoid arthritis: Report of a case. Calif Med 70(6): 459-463.
- Orozco Alcala J, Baum J (1973) Arthritis during sickle-cell crisis. The New England journal of medicine 288(8): 420.
- Schumacher HR, Dorwart BB, Bond J, Alavi A, Miller W (1977) Chronic synovitis with early cartilage destruction in sickle cell disease. Annals of the rheumatic diseases 36(5): 413-419.
- Espinoza LR, Spilberg I, Osterland CK (1974) Joint manifestations of sickle cell disease. Medicine (Baltimore) 53(4): 295-305.
- McFarlane IM OD, Pathiparampil J, Sanchez R, Levinson J, Barrett Campbell O, et al. (2017) Prevalence and Clinical Characteristics of Rheumatoid Arthritis in an Inner City Population with Sickle Cell Disease. Rheumatology (Sunnyvale) 7: 218.
- McInnes IB, Schett G (2011) The pathogenesis of rheumatoid arthritis. N Engl J Med 365(23): 2205-2019.
- Bridges SL, Hughes LB, Mikuls TR, Howard G, Tiwari HK, et al. (2003) Early rheumatoid arthritis in African-Americans: The CLEAR Registry. Clinical and experimental rheumatology 21(5 Suppl 31): S138-145.
- Bridges SL, Jenq G, Moran M, Kuffner T, Whitworth WC, et al. (2002) Single-nucleotide polymorphisms in tumor necrosis factor receptor genes: definition of novel haplotypes and racial/ethnic differences. Arthritis Rheum 46(8): 2045-2050.
- Tang Q, Danila MI, Cui X, Parks L, Baker BJ, et al. (2015) Expression of Interferon-gamma Receptor Genes in Peripheral Blood Mononuclear Cells Is Associated With Rheumatoid Arthritis and Its Radiographic Severity in African Americans. Arthritis Rheumatol 67(5): 1165-1170.
- Bridges SL, Causey ZL, Burgos PI, Huynh BQ, Hughes LB, et al. (2010) Radiographic severity of rheumatoid arthritis in African Americans: results from a multicenter observational study. Arthritis Care Res (Hoboken) 62(5): 624-631.
- Rees DC, Williams TN, Gladwin MT (2010) Sickle-cell disease. Lancet 376(9757): 2018-2031.
- Manwani D, Frenette PS (2013) Vaso-occlusion in sickle cell disease: pathophysiology and novel targeted therapies. Blood 122(24): 38923898.
- Zennadi R, Chien A, Xu K, Batchvarova M, Telen MJ (2008) Sickle red cells induce adhesion of lymphocytes and monocytes to endothelium. Blood 112(8): 3474-3483.
- (2017) Centers for Disease Control and Prevention. Data and Statistics.
- Paulukonis ST, Eckman JR, Snyder AB, Hagar W, Feuchtbaum LB, et al.(2016) Defining Sickle Cell Disease Mortality Using a Population-Based Surveillance System. Public health reports 131(2): 367-375.
- Maciaszek JL, Andemariam B, Huber G, Lykotrafitis G (2012) Epinephrine modulates BCAM/Lu and ICAM-4 expression on the sickle cell trait red blood cell membrane. Biophys J 102(5): 1137-1143.
- Perelman N, Selvaraj SK, Batra S, Luck LR, Erdreich Epstein A, et al. (2003) Placenta growth factor activates monocytes and correlates with sickle cell disease severity. Blood 102(4): 1506-1514.
- Hoppe C, Kuypers F, Larkin S, Hagar W, Vichinsky E, et al. (2011) A pilot study of the short-term use of simvastatin in sickle cell disease: effects on markers of vascular dysfunction. Br J Haematol 153(5): 655-663.
- Hunt BJ, Jurd KM (1998) Endothelial cell activation. A central pathophysiological process. BMJ 316(7141): 1328-1329.
- Pathare A, Al Kindi S, Alnaqdy AA, Daar S, Knox-Macaulay H, et al. (2004) Cytokine profile of sickle cell disease in Oman. Am J Hematol 77(4): 323328.
- Hounkpe BW, Fiusa MM, Colella MP, da Costa LN, Benatti Rde O, et al. (2015) Role of innate immunity-triggered pathways in the pathogenesis of Sickle Cell Disease: a meta-analysis of gene expression studies. Sci Rep 5:17822.
- Schaer DJ, Buehler PW, Alayash AI, Belcher JD, Vercellotti GM (2013) Hemolysis and free hemoglobin revisited: exploring hemoglobin and hemin scavengers as a novel class of therapeutic proteins. Blood 121(8): 1276-1284.
- Ataga KI, Kutlar A, Kanter J, Liles D, Cancado R, et al. (2017) Crizanlizumab for the Prevention of Pain Crises in Sickle Cell Disease. N Engl J Med 376(5): 429-439.
- Chantrathammachart P, Pawlinski R (2012) Tissue factor and thrombin in sickle cell anemia. Thromb Res 129(Suppl 2): S70-S72.
- Frelinger AL, Jakubowski JA, Brooks JK, Carmichael SL, Berny-Lang MA, et al. (2014) Platelet activation and inhibition in sickle cell disease (pains) study. Platelets 25(1): 27-35.
- Frenette PS (2002) Sickle cell vaso-occlusion: multistep and multicellular paradigm. Curr Opin Hematol 9(2): 101-106.
- Hidalgo A, Chang J, Jang JE, Peired AJ, Chiang EY,et al. (2009) Heterotypic interactions enabled by polarized neutrophil microdomains mediate thromboinflammatory injury. Nat Med 15(4): 384-391.
- Lim MY, Ataga KI, Key NS (2013) Hemostatic abnormalities in sickle cell disease. Curr Opin Hematol 20(5): 472-477.
- Looney MR, Matthay MA (2009) Neutrophil sandwiches injure the microcirculation. Nat Med 15(4): 364-366.
- Brennan FM, McInnes IB (2008) Evidence that cytokines play a role in rheumatoid arthritis. J Clin Invest 118(11): 3537-3545.
- Koch AE (1998) Review: angiogenesis: implications for rheumatoid arthritis. Arthritis Rheum 41(6): 951-962.
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S (2007) Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol 7(9): 678-689.
- Filippin LI, Vercelino R, Marroni NP, Xavier RM (2008) Redox signalling and the inflammatory response in rheumatoid arthritis. Clin Exp Immunol 152(3): 415-422.
- McInnes IB, Leung BP, Field M, Wei XQ, Huang FP, et al. (1996) Production of nitric oxide in the synovial membrane of rheumatoid and osteoarthritis patients. J Exp Med 184(4): 1519-1524.
- McFarlane IM, Saperstein Y, Rodriguez-Alvarez M, Zhaz Leon S, Koci K, et al. (2017) Rheumatoid arthritis in sickle-cell population: pathophysiologic insights, clinical evaluation and management. Rheumatology (Sunnyvale) 7(3) pii: 225.
- Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, et al. (2010) 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 69(9): 1580-1588.
- Darbari DS, Castro O, Taylor JGt, Fasano R, Rehm J, et al. (2008) Severe vaso-occlusive episodes associated with use of systemic corticosteroids in patients with sickle cell disease. J Natl Med Assoc 100(8): 948-951.
- Gladman DD, Bombardier C (1987) Sickle cell crisis following intraarticular steroid therapy for rheumatoid arthritis. Arthritis Rheum 30(9): 1065-1068.
- Griffin TC, Mclntire D, Buchanan GR (1994) High-dose intravenous methylprednisolone therapy for pain in children and adolescents with sickle cell disease. N Engl J Med 330(11): 733-737.
- Huang JC, Gay R, Khella SL (1994) Sickling crisis, fat embolism, and coma after steroids. Lancet. 344(8927): 951-952.
- Shapiro MP, Hayes JA (1984) Fat embolism in sickle cell disease. Report of a case with brief review of the literature. Arch Intern Med 144(1): 181-182.
- Strouse JJ, Hulbert ML, DeBaun MR, Jordan LC, Casella JF (2006) Primary hemorrhagic stroke in children with sickle cell disease is associated with recent transfusion and use of corticosteroids. Pediatrics 118(5): 19161924.
- http://www. ehealthme.com/drug-interaction/plaquenil/ hydroxyurea/.j
- Brandalise SR, Assis R, Laranjeira ABA, Yunes JA, de Campos-Lima PO(2017) Low-dose methotrexate in sickle-cell disease: a pilot study with rationale borrowed from rheumatoid arthritis. Exp Hematol Oncol 6:18.
- Cordner S, De Ceulaer K (2003) Musculoskeletal manifestations of hemoglobinopathies. Curr Opin Rheumatol 15(1): 44-47.
- Singh JA, Saag KG, Bridges SL, Akl EA, Bannuru RR, et al. (2016) 2015 American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol 68(1): 1-26.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






