- Submissions

Full Text
Polymer Science: Peer Review Journal
Assessing Particle Size and Surface Charge in Drug Carrier Nanoparticles for Enhanced Cancer Treatment: A Comprehensive Review Utilizing DLS and Zeta Potential Characterization
Mohammad Hossein Karami* and Majid Abdouss*
Department of Chemistry, Amirkabir University of Technology, Iran
*Corresponding author:Mohammad Hossein Karami and Majid Abdouss, Department of Chemistry, Amirkabir University of Technology, P O Box 15875- 4413, Tehran, Iran
Submission: December 18, 2023;Published: February 08, 2024

ISSN: 2770-6613 Volume5 Issue3
Abstract
The provided information underscores the significance of cancer control, including early detection, treatment, and prevention. The World Health Organization (W.H.O) is actively working towards improving access to screening, prevention, and treatment services, as well as raising awareness about the importance of cancer prevention and early detection. Researchers are also exploring the potential of natural substances to develop safer drugs for cancer treatment, with the W.H.O providing support to organizations involved in this research. One approach being explored is the use of Drug Delivery Systems (DDSs) to minimize damage to healthy tissue during cancer treatment. Maintaining a Zeta potential range between -30 and +30mV is considered optimal for nanocarriers in the bloodstream, as it ensures stability and long-term activity. Additionally, nanoparticles with a size below 200nm can be effectively distributed in the body and have an increased penetration rate into cancer cells. Smaller particles exhibit better mobility and can more efficiently reach their targets. A recent study analyzed the particle size and surface charge of newly designed drug nanocarriers to improve cancer treatment. This promising approach aims to optimize drug delivery and enhance the efficacy of cancer therapy.
Keywords:Cancer; Nanocarriers; Dynamic Light Scattering (DLS); Particle Size; Surface Charge Potential
Introduction
Cancer is a disease caused by abnormal cell growth that can affect anyone. It is a major global health concern and the second leading cause of death worldwide. The World Health Organization is working to reduce the incidence of cancer by promoting prevention, early detection, and access to treatment. The organization is also focused on improving cancer care in low- and middle-income countries, where access to cancer care is often limited. Research efforts are ongoing to develop new and innovative cancer treatments [1]. This highlights the critical need for effective prevention and treatment strategies to improve patient outcomes. While current strategies like medical procedures, radiation treatment, and chemotherapy have limitations and may not be entirely safe or effective, there has been a growing interest in identifying natural substances with effective compounds to develop low-risk drugs for cancer treatment. o address this, researchers have proposed using Drug Delivery Systems (DDSs) to deliver drugs directly to cancer cells while minimizing damage to healthy tissue [2,3]. pH-responsive systems have been developed to selectively release drugs in the lower pH environment of tumor sites, which improves the selectivity of anti-cancer drugs and minimizes side effects and overdosage. Additionally, these systems can enhance the accumulation of drugs in tumor sites due to the EPReffect [4]. The EPR (enhanced permeability and retention) effect is a phenomenon observed in tumors where the tumor vasculature is characterized by leaky and poorly organized blood vessels, leading to a higher permeability of nanoparticles and other macromolecules [5]. The EPR effect allows for selective accumulation of nanoparticles in tumor tissues due to the enhanced permeability of the tumor vasculature and the reduced lymphatic drainage, leading to an increased retention of the nanoparticles. Nanoparticles with sizes ranging from 10 to 200nm can accumulate in tumor tissue through the EPR effect, while avoiding normal tissues. This property has been used to develop targeted drug delivery systems for cancer treatment, where drugs are attached to nanoparticles and delivered directly to the tumor tissue, increasing their effectiveness and reducing toxicity to healthy tissues. This effect has been extensively studied and exploited for the development of nanocarriers for cancer drug delivery [6]. By taking advantage of the EPR effect, drug-loaded nanoparticles can selectively accumulate in the tumor tissue, leading to higher drug concentrations at the tumor site and a reduced systemic toxicity. The EPR effect has limitations, including inter- and intra-tumoral heterogeneity, which can affect the magnitude of the effect [7]. However, the EPR effect remains a promising approach for improving the efficacy of cancer treatment and has led to the development of several nanocarriers that are currently in clinical trials.
Nano drug delivery using nanoparticles in DDSs offers a promising alternative to traditional chemotherapy for breast cancer treatment. It allows for targeted drug delivery, reducing the risk of toxicity to healthy cells and tissues. Nanoparticles can release drugs in a controlled manner, increasing drug action duration and reducing the frequency of dosing. Preclinical studies have shown their effectiveness in treating breast cancer, and clinical trials are underway to test their safety and efficacy in humans. The use of DDSs in breast cancer treatment has the potential to improve patient outcomes and quality of life [8]. This approach involves engineering nanoparticles to target specific cells or tissues, which can improve drug efficacy and reduce side effects. One type of nanoparticle used in drug delivery is nanocarriers, which are typically made of biodegradable polymers or lipids [9]. Nanocarriers can encapsulate drugs, protecting them from degradation and improving their solubility, bioavailability, and circulation time in the body. They can also be designed to release drugs in a controlled manner, which can further enhance their efficacy while minimizing side effects [10]. Nanocarriers have shown promise in treating various diseases, including cancer, infectious diseases, and inflammatory disorders, and are being actively researched as a potential alternative to traditional drug delivery methods. Measuring the size and surface charge of nanoparticles is crucial for understanding their behavior in drug delivery [11]. Dynamic Light Scattering (DLS) is a method that is frequently employed in nanotechnology to determine the size of nanoparticles, including nanocarriers. The technique relies on measuring the intensity of light that is scattered by the particles as they move randomly in a liquid medium due to Brownian motion. This information is important for optimizing drug delivery as particle size can impact their ability to penetrate cells and tissues and their circulation time in the body [12]. Surface charge is another crucial factor in drug delivery as it can influence the interaction of nanoparticles with cells and tissues. Measuring the zeta potential of nanoparticles, which is the electrical potential difference between the particle surface and the surrounding solution, can determine their surface charge. DLS can also be used to measure zeta potential, providing information to optimize nanocarrier performance in drug delivery. In summary, determining the size and surface charge of nanocarriers using DLS is an essential factor in drug release. This information can be used to improve drug delivery and enhance the safety and efficacy of nanocarrier-based drug therapies [13].
In summary, a recent study examined the particle size and surface charge of newly developed drug nanocarriers for cancer treatment. This investigation aimed to optimize drug delivery and improve the efficacy of cancer therapy. By analyzing these parameters, researchers can potentially enhance the effectiveness of cancer treatment and provide a promising solution in the field of oncology. This research is the first to examine the particle size and surface charge of manufactured nanocarriers. By studying these parameters, the researchers aim to understand how they affect drug delivery and their potential impact on cancer treatment. This unique approach provides valuable insights for improving the design of nanocarriers to enhance therapeutic outcomes.
Investigation of Particle Size and Surface Charge in New Nanocarriers
The chitosan-agarose nanocomposite hydrogel containing nanoclay designed by Yazdian et al. has shown promising results in improving the release of the anticancer drug curcumin in a breast cancer cell line. The DLS test results indicate that the particle size of the nanocomposite falls within the range of 340-460 nanometers, and the dispersion index (PDI) is 0.2, which indicates mono-dispersity and is suitable for drug release. Additionally, the Zeta potential test showed a surface charge of 47mV, indicating the stability of the nanocarrier. These findings suggest that the chitosan-agarose nanocomposite hydrogel containing nanoclay has the potential for breast cancer treatment [14].
Hascicek and colleagues developed a chitosan-based nanocarrier that contains bovine serum albumin nanoparticles for the release of methotrexate, an antitumor drug used to treat breast cancer. Their study found that the size of the particles in the nanocarrier ranged from 234.1 to 733.1nm, and the dispersion index ranged from 0.294 to 0.583. The presence of chitosan reduced the particle size, which may be attributed to the increased network of albumin nanoparticles that enhance the stability of the nanocarrier. However, using small amounts of chitosan particles (12 and 24mg) led to particle clumping. To regulate the biological behavior of drug release from the nanocarrier, the size of the surface charge was measured. The surface charge was found to be 38.70mV, which can increase the repulsive force to prevent particle clumping. These findings suggest that this chitosan-based nanocarrier has the potential to deliver anticancer drugs effectively for breast cancer treatment [15].
Ghadami and colleagues conducted a study on chitosan nanocarriers containing third-type iron oxide nanoparticles (Fe3O4) and graphene oxide nanoparticles to enhance the release of curcumin, an anticancer drug, in MCF-7 cell lines (Breast Cancer). The findings of their study revealed that the size of the nanocarrier ranged from 860 to 910nm, and the dispersion index was 0.4, which is acceptable for improving drug release from nanocarriers. The surface charge of the nanocarrier ranged between -34 and -37.3mV, indicating the stability of the designed nanocarrier. These results suggest that chitosan nanocarriers containing Fe3O4 and graphene oxide nanoparticles have the potential to improve the release of curcumin in breast cancer treatment [16].
Rashedi and colleagues developed a chitosan nanocarrier containing agarose nanoparticles to enhance breast cancer treatment. Initially, they loaded curcumin and 5-fluorouracil (5- FU), two anticancer drugs, in the nanocarrier for simultaneous release. Their study revealed that the size of the nanocarrier was 341 nanometers, and the dispersion index of the particles was 1.07. The zeta potential value for the nanocarrier was -33.1mV. These findings suggest that the chitosan nanocarrier containing agarose nanoparticles has the potential to improve the simultaneous release of multiple anticancer drugs for breast cancer treatment [17].
The presence of negative surface charge in nanoparticles prevents them from being absorbed by plasma proteins, resulting in their removal from macrophages [17]. Macrophages play a crucial role in identifying and engulfing cancer cells, dead cells, and microbes in the human body. The zeta potential range between -30 and +30mV is ideal for designing nanocarriers because it ensures their stability in the bloodstream and prolongs their circulation time, as depicted in Figure 1. Rashedi and colleagues developed chitosan nanocarriers that contain halloysite nanotubes and carbon nanotubes to improve breast cancer treatment. They loaded the anticancer drug curcumin into the nanocarrier. The DLS results, showed a narrow and sharp peak, indicating mono scattering. The size of the nanocarrier was found to be 267.37, suggesting that the chitosan nanocarriers containing halloysite nanotubes and carbon nanotubes have the potential to improve the delivery of curcumin for breast cancer treatment. The zeta potential for chitosan nanocarriers containing halloysite nanotubes and carbon nanotubes investigated. The results indicate that the surface charge is 32.5mV, suggesting that the nanocarrier is stable. It is worth noting that an electrostatic repulsion force of more than 30mV is necessary to ensure the stability of the nanocarrier. Therefore, the chitosan nanocarriers containing halloysite nanotubes and carbon nanotubes have the potential to be a stable for breast cancer treatment [18].
Figure 1:Schematic curve of zeta potential of chitosan nano carriers containing agarose nanoparticles and drugs.
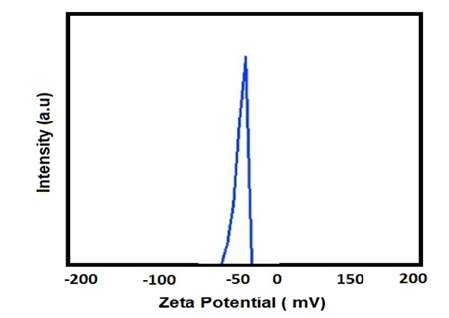
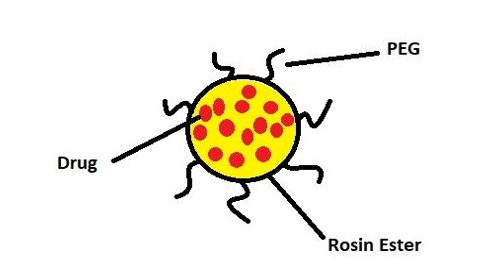
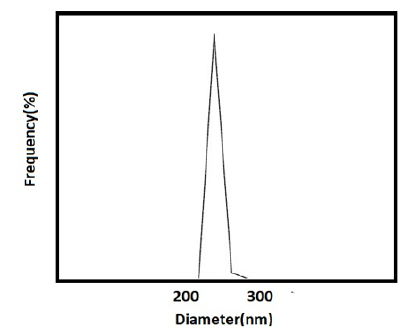
Kalındemirtas and colleagues developed a polyethylene glycol nanocarrier containing rosin ester nanoparticles to enhance breast cancer treatment by improving the release of 5-fluorouracil and Carmofur drugs (Figure 2). The results showed that the loading of two different drugs altered the size of the nanocarrier. Specifically, the size of the nanocarrier particles containing 5-fluorouracil and Carmofur drugs was 197.9 and 182.8 nanometers, respectively.
The zeta potential value for both nanocarriers was 0.7mV with negative polarity. Furthermore, the dispersion index for the nanocarrier containing 5-fluorouracil was 0.203, while it was 0.145 for the nanocarrier containing Carmofur. These findings suggest that the polyethylene glycol nanocarrier containing rosin ester nanoparticles to enhance the release of multiple anticancer drugs for breast cancer treatment [19].
Figure 2:Schematic of the structure of polyethylene glycol nanocarrier containing ester resin nanoparticles and drug.
Jayakanan and colleagues developed a fluorescent nanocarrier (Fluorescent ABC-Triblock) that contains the anticancer drug Cisplatin to enhance the treatment of breast and neck cancer. The study found that the size of the nanocarrier was 160 nanometers, which was consistent with the results obtained from the SEM. These findings suggest that the fluorescent nanocarrier could be a promising for the treatment of breast and neck cancer [20]. Yazdian and colleagues developed a nano hydrogel containing chitosan, carbon quantum dot, and aptamer to enhance the release of the anticancer drug 5-fluorouracil in breast cancer cell lines. The study found that the average size of the nanocarrier particles was 122.7 nanometers, which is promising because particles smaller than 200nm can improve distribution within the system and the human body and penetrate cancerous tumor tissue more effectively. These results suggest that the hydrogel could be a promising drug for the treatment of cancer [21].
Guo and colleagues developed a hybrid nanocarrier containing carbon nanocages and nanochitosan for the release of 5-fluorouracil. The study found that the size of the nanocarrier was 960.8 nanometers, which is relatively large for a nanocarrier. This could potentially limit its ability to penetrate tumor tissues and distribute evenly throughout the system. In contrast, Jin and colleagues investigated a nanocarrier containing nano gold (core) and polyN-isopropylacrylamide-co-methacrylic acid polymer (shell) for the release of 5-fluorouracil. The study found that the average size of the nanocarrier particles was 195 nanometers, which is within the ideal range for effective drug delivery. These results suggest that the nanocarrier containing nano gold and polyN-isopropylacrylamide-co-methacrylic acid polymer could be a ideal system for the treatment of cancer [22]. Sabouni and colleagues prepared and investigated a liposome nanocarrier containing nanoparticles of organometallic framework type III iron nanoparticles (benzene tricarboxylate) for the release of the antitumor drug doxorubicin. The study found that the size of the nanoparticles alone was 287.3 nanometers with a dispersion index of 0.206.
However, when liposomes were added to the nanocarrier, the size of the nanocarrier decreased to 1.163 nanometers with a dispersion index of 0.090. This indicates that the presence of liposomes made the nanocarrier smaller and prevented agglomeration. The average size of the nanocarrier is within the ideal range for effective drug delivery, as larger nanoparticles can be cleared by the immune system before reaching the cancer tumor site [23]. Nanoparticles that are larger than 200nm can be recognized and cleared by the immune system before they reach the cancer tumor site. This is because the immune system sees them as foreign and potentially harmful and tries to eliminate them. Therefore, nanoparticles that are smaller than 200nm are preferred for drug delivery, as they can evade the immune system and reach the tumor site more effectively. The use of liposome nanocarriers containing organometallic framework type III iron nanoparticles with an average size of 150nm is a good approach for drug delivery [24].
Rahafar and colleagues investigated the use of polyvinyl pyrrolidone and polyvinyl alcohol nanocarriers containing titanium oxide nanoparticles for the release of quercetin in fibroblast (L929) and U87 cell lines [25]. The study found that the size of the nanocarriers made of polyvinyl pyrrolidone and polyvinyl alcohol was 119 nanometers with a surface charge of 31 millivolts. The addition of titanium oxide nanoparticles to the nanocarrier increased the size of the particles to 300 nanometers, with a surface charge of 7 millivolts. However, after adding the drug to the nanocarrier, the size of the nanocarrier increased to 330nm with a zeta potential of 51mV. A zeta potential above 30mV is ideal as it creates electrostatic repulsion and prevents particles from clumping together. The increase in size with each step of adding nanoparticles to the nanocarrier indicates that the nanocarrier synthesis was performed correctly. Overall, the use of polyvinyl pyrrolidone and polyvinyl alcohol nanocarriers containing titanium oxide nanoparticles could be an ideal approach for the delivery of quercetin in cancer cell lines, particularly in glioblastoma research [26].
Yazdian and colleagues investigated the use of chitosan nanocarrier, polyvinylpyrrolidone, and iron oxide nanoparticles for the release of the anticancer drug doxorubicin in breast cancer cell lines (Figure 3). The study found that the size of the nanocarrier particles was 247 nanometers with a zeta potential of 31.2mV. A zeta potential higher than 30mV shows the stability of the nanocarrier as it prevents the nanoparticles from clumping together. The study also found that the particle distribution was very narrow or monodisperse and was relatively uniform, which is important for effective drug delivery. Overall, the use of chitosan nanocarrier, polyvinylpyrrolidone, and iron oxide nanoparticles could be an ideal approach for the delivery of doxorubicin in cell lines [27].
Figure 3:Schematic curve of the size of nano particles carrying chitosan, polyvinyl pyrrolidone and iron oxide nanoparticles for the release of doxorubicin drug.
Yazdian and colleagues developed a nanocarrier made of pyrrolidone, hydroxyapatite, and agarose for delivering the drug quercetin to breast cancer cell lines. The nanocarrier had a size range of 440-536 nanometers and a dispersion index of 0.4, indicating its suitability for drug release. The nanocarrier also had a negative surface charge of -28.1 to -29.5, indicating its stability. This nanocarrier could be a promising approach for delivering quercetin to breast cancer cells [28]. Yazdian and colleagues developed a chitosan nanocarrier containing polyvinylpyrrolidone and alumina nanoparticles for the release of doxorubicin in MCF-7 cell lines. The nanocarrier had an average particle size of 141 nanometers and a dispersion index of 0.12, indicating almost mono dispersion. The zeta potential was -47mV, indicating the stability of the nanocomposite [29]. The interaction of nanocomposites or nanocarrier with the cell membrane plays a vital role in determining their biocompatibility. In this regard, negatively charged nanocomposites have been found to be more biocompatible than positively charged ones. Moreover, these nanocomposites also exhibit a longer retention time in the bloodstream. These findings indicate that the use of negatively charged nanocomposites could be ideal strategy for the controlled release of doxorubicin in the cell [30].
In their study, Diez-Pascual and colleagues investigated a polyacrylic acid nanocarrier containing polyvinylpyrrolidone, gamma alumina nanoparticles, and the anti-cancer drug quercetin in breast cancer cell lines. The nanocarrier had an average size of 402.4nm and a dispersion index of 0.24 [31]. The double emulsion method used to fabricate the nanocarrier reduced the particle size. A dispersion index below 0.3 is considered suitable for nanocarrier design, and in this case, only one peak was observed in the diagram, indicating mono dispersion. The zeta potential value was 34.9mV, indicating the stability of the nanocarrier. The use of Span 80, a surfactant, increased the stability of the nanocarrier in this research [32].
Rashedi and colleagues investigated a chitosan (CS) nanocarrier containing nano alumina and iron oxide nanoparticles for the anticancer drug 5-fluorouracil in breast cancer cell lines. The nanocarrier had a monodispersion structure and an average hydrodynamic diameter of 468.3 nanometers, which is suitable for drug delivery applications. The zeta potential was 48.24mV, indicating the stability of the nanocarrier. The dispersion index was 0.5, indicating proper distribution of the fabricated nanocarrier. The high zeta potential value was attributed to the presence of amine in the CS structure, indicating appropriate coating of the chitosan for nanocarriers. This study suggests that the chitosan nanocarrier containing nano alumina and iron oxide nanoparticles could be an ideal approach for delivering 5-fluorouracil in MCF-7[33].
In their study, Tiwari and colleagues investigated a nanocarrier containing multi-walled carbon nanotubes and carboxyhydrate ligand for the release of doxorubicin (MCF-7). The size of the nanocarrier was dependent on the type of carbohydrate used, with galactose resulting in a size of 204nm, mannose resulting in a size of 171nm, and lactose resulting in a size of 157nm. The dispersion index of the nanocarrier was 0.21, 0.23, and 0.24 for galactose, mannose, and lactose, respectively. The zeta potential value was 19.7mV for galactose, and 16.6 and 15.9mV for mannose and lactose, respectively. These results suggest that the nanocarrier containing multi-walled carbon nanotubes and carboxyhydrate ligand could be a promising approach for the release of doxorubicin in breast cancer cell lines, with the size and surface charge of the nanocarrier being dependent on the type of carbohydrate used [34].
Yazdian and colleagues conducted a study on the use of chitosan nanocarriers, nanoclay, and nitrogen-containing carbon quantum dot nanoparticles as a means of delivering the anticancer drug doxorubicin to breast cancer cells. The study found that the nanocarrier had a size of 276.80 nanometers and a monodisperse distribution. When the nanocarrier was of the same size and shape, the scattering distribution was of a single type. The surface charge, or zeta potential, was measured at 31.5mV, indicating the stability of the nanocarrier. After one month, there was no sign of lumpiness in the sample, indicating that the positive zeta potential improved drug absorption in the body [35]. In their research, Pourmadadi et al. focused on improving the release of the anticancer drug curcumin in brain cancer cells using chitosan nanocarriers that contained gelatin and carbon quantum dot nanoparticles. Their findings indicated that the nanocarrier had a size of 216.8 nanometers and a sharp peak. The zeta potential value of the nanocarrier ranged between 25 and 75mV, which is considered suitable for designing nanocarriers since it is higher than 20 and indicates that the nanocarrier is both stable and has a proper distribution [36].
In their study, Pourmadadi et al. used nanocarriers containing carboxymethyl cellulose, gelatin, and organometallic framework nanoparticles to enhance the release of quercetin in breast cancer cells. The nanocarriers had a negative surface charge of -40.1mV, were stable, and had an average particle size of 250nm. The study suggests that these nanocarriers could be an effective approach for delivering quercetin to breast cancer cells, due to their small particle size and negative surface charge. he zeta potential analysis showed that the nanocarriers had a negative surface charge of -40.1mV, indicating their stability. The absolute value of zeta was above 30mV, which further confirms the stability of the nanocarriers. This suggests that the nanocarriers are less likely to aggregate and are more likely to remain stable in biological environments [37-40].
In their study, Pascual et al. investigated a nanocarrier consisting of carboxymethyl cellulose, starch with reduced graphene oxide nanoparticles, and curcumin for breast cancer treatment. The nanocarrier had a size range of 247-455 nanometers with a dispersion index of 0.23, indicating monodispersion. The use of Span 80 surfactant in the double emulsion method increased the size of the nanocarrier, creating a membrane-like layer that enhanced stability. The nanocarrier exhibited a surface charge ranging from negative 7.8 to positive 82.4mV, with an average of -52.6mV, indicating stability due to the presence of the surfactant. This is in contrast to other studies where the use of surfactant 80 resulted in a negative zeta potential [40-44]. Pascual and colleagues investigated a nanocarrier containing polyvinyl pyrrolidone, gelatin with graphene oxide nanoparticles and quercetin drug for breast cancer treatment. The size of the nanocarrier was 468 nanometers, with an increase in the hydrodynamic radius observed with each added material, indicating proper synthesis and preparation [45]. The size of the nanocarrier was suitable for drug release. The zeta potential of the nanocarrier was negative 40mV, indicating stability due to the negative surface charge of different layers. This negative zeta potential also enhances biocompatibility and prolongs the nanocarrier’s presence in the bloodstream [46-50].
Nanocarrier chitosan, nanoclay, and nanoparticles of iron oxide were investigated for the release of the anticancer drug quercetin in breast cancer cell line. The size of the nanocarrier is 161.3 nanometers and also The value of zeta potential is equal to 53Mv. The purportedly significant intrinsic worth is credited to the existence of amino functionalities in chitosan, which facilitates the formation of an appropriate layer for the nanocarrier [51-53]. Parsi and colleagues investigated a chitosan nanocarrier, nanohalosite, containing graphite carbon nitride nanoparticles for the release of the anticancer drug quercetin in breast cancer cells. The nanocarrier had a size of 454.65 nanometers, and a dispersion index of 0.4, indicating suitable dispersion for drug release applications. The zeta potential was 55.23mV, indicating high stability due to electrostatic repulsion forces. Zeta potentials above +30mV prevent particle oscillation and clumping, while values below 30mV tend to cause particle agglomerating [54].
Yazdian and colleagues investigated a chitosan-agarose nanocarrier containing gamma alumina nanoparticles for the release of the 5-fluorouracil (MCF-7). The nanocarrier had a size of 272 nanometers, and a zeta potential value of 67 millivolts. A zeta potential value above 30mV is considered quite stable, and sufficient electrostatic repulsion ensures that particle aggregation will not be a problem for the nanocarrier [55-60].
In their study, Karami et al. utilized DLS analysis to assess the size distribution and polydispersity of the nanoparticles. The nanoparticles had an average diameter of 227.3nm, ranging from 160 to 320nm. The low polydispersity value of 0.24 suggests a monodisperse system well-suited for drug delivery. The zeta potential measurements revealed a mean value of -37.85mV, indicating excellent stability of the nanocarrier. The negative charge of the CUR drug enhances its biocompatibility and facilitates efficient clearance by macrophages. Furthermore, the slow movement of the nanocarriers in the bloodstream makes them highly suitable for drug delivery applications [61]. Yazdian and colleagues designed a chitosan-agarose nanocarrier containing iron oxide nanoparticles and loaded it with the drug curcumin for research on drug release in breast cancer cells. The nanocarrier had a size of 279 nanometers, indicating potential for easy transport and homogeneous mixing. The zeta potential was 48mV, indicating stability in the bloodstream [58-61].
Conclusion
Zeta potential is a crucial parameter that measures the surface charge of nanoparticles and influences their stability and behavior in the body. For designing nanocarriers, a zeta potential range of -30 to +30mV is considered ideal as it provides stability and longevity in the bloodstream. This ensures that nanocarriers remain intact and do not aggregate or break down, maximizing their effectiveness. Some studies suggest that a zeta potential exceeding 30mV contributes to the stability of nanocarriers by creating electrostatic repulsion forces that prevent particle clumping. Particle size is another important factor in nanocarrier design [62]. Nanocarriers with a size below 200nm can enhance drug distribution and penetration into cancerous tumor tissue. Smaller particles can more easily navigate through the bloodstream and reach their targeted areas. Negatively charged nanocarriers are believed to have prolonged circulation in the bloodstream compared to positively charged ones. The negative charge slows down their movement, allowing for extended interaction with the body. Surfactant 80, commonly used in nanocarrier production, has been found to generate a negative zeta potential in many studies. Utilizing Surfactant 80 can thus increase the stability of nanocarriers by promoting a negative zeta potential. In conclusion, the research outcomes indicate that the examination of treatment methods, particularly the utilization of nanocarriers for drug delivery, has the potential to significantly enhance cancer treatment. The development of monodisperse nanocarriers with desirable characteristics, such as a narrow size distribution and good stability, offers a promising avenue for improving treatment outcomes. By effectively delivering anticancer drugs, such as CUR, these nanocarriers can enhance treatment efficacy while minimizing adverse effects. Additionally, the ability of nanocarriers to efficiently navigate through the bloodstream enables targeted drug delivery, further enhancing the effectiveness of cancer treatment. Therefore, through the exploration and implementation of innovative treatment methods like nanocarriers, cancer treatment can be enhanced by improving drug delivery, minimizing side effects, and optimizing therapeutic outcomes.
Author Contributions Statement
Mohammad Hossein Karami: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Resources, Data Curation, Writing - Original Draft, Writing - Review & Editing, Visualization, Supervision, Project administration, Methodology. Majid Abdouss: Conceptualization, Validation, Editing, Supervision.
References
- Ostovar S, Pourmadadi M, Shamsabadipour A, Mashayekh P (2023) Nanocomposite of chitosan/gelatin/carbon quantum dots as a biocompatible and efficient nanocarrier for improving the curcumin delivery restrictions to treat brain cancer. Int J Biol Macromol 242(Pt 3): 1234-1245.
- Pourmadadi M, Darvishan S, Abdouss M, Yazdian F, Rahdar A, et al. (2023) pH-responsive polyacrylic acid (PAA)-carboxymethyl cellulose (CMC) hydrogel incorporating halloysite nanotubes (HNT) for controlled curcumin delivery. Ind Crops Prod 197: 116654.
- Parvaneh S, Pourmadadi M, Abdouss M, Pourmousavi SA, Yazdian F, et al. (2023) Carboxymethyl cellulose/starch/reduced graphene oxide composite as a pH-sensitive nanocarrier for curcumin drug delivery. Int J Biol Macromol 241: 124566.
- Norouzi Z, Abdouss M (2023) Preparation and characterization of β-cyclodextrin grafted chitosan nanofibers via electrospinning for dye removal. Polym Bull 81: 3641-3677.
- Darvishan S, Pourmadadi M, Abdouss M, Mazinani S, Yazdian F, et al. (2023) Gamma alumina coated-PAA/PVP hydrogel as promising quercetin nanocarrier: Physiochemical characterization and toxicity activity. J Drug Deliv Sci Technol 84: 104500.
- Cukierman E, Khan DR (2010) The benefits and challenges associated with the use of drug delivery systems in cancer therapy. Biochem Pharmacol 80(5): 762-770.
- Allen TM, Cullis PR (2004) Drug delivery systems: entering the mainstream. Science 303(5665): 1818-1822.
- Kamaly N, Yameen B, Wu J, Farokhzad OC (2016) Degradable controlled-release polymers and polymeric nanoparticles: mechanisms of controlling drug release. Chem Rev 116(4): 2602-2663.
- Sharma AK, Garg T, Goyal AK, Rath G (2016) Role of microemulsions in advanced drug delivery. Artif Cells Nanomed Biotechnol 44(4): 1177-1185.
- Nezhad-Mokhtari P, Ghorbani M, Roshangar L, Soleimani Rad J (2019) A review on the construction of hydrogel scaffolds by various chemically techniques for tissue engineering. Eur Polym J 117: 64-76.
- Gurumurthy B, Janorkar AV (2021) Improvements in mechanical properties of collagen-based scaffolds for tissue engineering. Curr Opin Biomed Eng 17: 100253.
- Unhoz AH, de Paiva H, de Miranda LF, De Oliveira EC, Cons Andrades R, et al. (2015) Synthesis and characterization of pseudoboehmite and gamma-alumina. Mater Sci Forum 820: 131-136.
- Eftekhari A, Jahanbani Y, Shafiee S, Davaran S, Roshangar L, et al. (2022) Stem cells technology as a platform for generating reproductive system organoids and treatment of infertility-related diseases. Cell Biol Int 46(4): 512-522.
- Peng CA, Kozubowski L, Marcotte WR (2020) Advances in plant-derived scaffold proteins. Front Plant Sci 11: 122.
- Chinta ML, Velidandi A, Pabbathi NPP, Dahariya S, Parcha SR (2021) Assessment of properties, applications and limitations of scaffolds based on cellulose and its derivatives for cartilage tissue engineering: A review. Int J Biol Macromol 175: 495-515.
- Iravani S, Varma RS (2019) Plants and plant-based polymers as scaffolds for tissue engineering. Green Chem 21(18): 4839-4867.
- Indurkar A, Pandit A, Dandekar P, Jain R (2021) Plant-based biomaterials in tissue engineering. Bioprinting 21: e00127.
- Ľudmila H, Michal J, Andrea Š, Aleš H (2015) Lignin, potential products and their market value. Wood Res 60(6): 973-986.
- Gupta PK, Raghunath SS, Prasanna DV, Venkat P, Shree V, Chithananthan C, et al. (2019) An update on overview of cellulose, its structure and applications. In: Pascual AR, Martín MEE (Eds.), Cellulose.
- Wsoo MA, Shahir S, Mohd Bohari SP, Nayan NHM, Razak SIA (2020) A review on the properties of electrospun cellulose acetate and its application in drug delivery systems: a new perspective. Carbohydr Res 491: 107978.
- Liu R, Dai L, Xu C, Wang K, Zheng C, et al. (2020) Lignin-based micro and nanomaterials and their composites in biomedical applications. ChemSusChem 13(17): 4266-4283.
- Spiridon I (2018) Biological and pharmaceutical applications of lignin and its derivatives: A Mini-Review. Cellulose Chem Technol 52(7- 8): 543-550.
- Roshangar L, Rad JS, Kheirjou R, Khosroshahi AF (2021) Using 3D-bioprinting scaffold loaded with adipose-derived stem cells to burns wound healing. J Tissue Eng Regen Med 15(6): 546-555.
- Li YY, Wang B, Ma MG, Wang B (2018) Review of recent development on preparation, properties, and applications of cellulose-based functional materials. Int J Polym Sci pp: 1-18.
- Carrion CC, Nasrollahzadeh M, Sajjadi M, Jaleh B, Soufi GJ, et al. (2021) Lignin, lipid, protein, hyaluronic acid, starch, cellulose, gum, pectin, alginate and chitosan-based nanomaterials for cancer nanotherapy: challenges and opportunities. Int J Biol Macromol 178: 193-228.
- Karami M H, Abdouss M (2024) Recent advances of carbon quantum dots in tumor imaging. Nanomed J 11(1): 13-35.
- Unni R, Varghese R, Bharat Dalvi Y, Augustine R, MS L, et al. (2022) Characterization and in vitro biocompatibility analysis of nanocellulose scaffold for tissue engineering application. J Polym Res 29(8): 358.
- Madni A, Kousar R, Naeem N, Wahid F (2021) Recent advancements in applications of chitosan-based biomaterials for skin tissue engineering. J Bioresour Bioprod 6(1): 11-25.
- Hickey RJ, Pelling AE (2019) Cellulose biomaterials for tissue engineering. Front Bioeng Biotechnol 7: 45.
- Dugan JM, Gough JE, Eichhorn SJ (2013) Bacterial cellulose scaffolds and cellulose nanowhiskers for tissue engineering. Nanomedicine (Lond) 8(2): 287-298.
- Mohite BV, Patil SV (2014) A novel biomaterial: bacterial cellulose and its new era applications. Biotechnol Appl Biochem 61(2): 101- 110.
- Duval A, Lawoko M (2014) A review on lignin-based polymeric, micro-and nano-structured materials. React Funct Polym 85: 78-96.
- Chio C, Sain M, Qin W (2019) Lignin utilization: a review of lignin depolymerization from various aspects. Renewable Sustainable Energy Rev 107: 232-249.
- Beckham GT, Johnson CW, Karp EM, Salvachúa D, Vardon DR (2016) Opportunities and challenges in biological lignin valorization. Curr Opin Biotechnol 42: 40-53.
- Liang R, Zhao J, Li B, Cai P, Loh XJ, et al. (2020) Implantable and degradable antioxidant poly(ε-caprolactone)-lignin nanofiber membrane for effective osteoarthritis treatment. Biomaterials 230: 119601.
- Karami MH, Abdouss M, Karami M (2023) Evaluation of in vitro and ex vivo models for studying the effectiveness of vaginal drug systems in controlling microbe infections: A systematic review. Clin J Obst Gynecol 6: 201-215.
- Mahendiran B, Muthusamy S, Selvakumar R, Rajeswaran N, Sampath S, et al. (2021) Decellularized natural 3D cellulose scaffold derived from Borassus flabellifer () as extracellular matrix for tissue engineering applications. Carbohydr Polym 272: 118494.
- Subia B, Kundu J, Kundu SC (2010) Biomaterial scaffold fabrication techniques for potential tissue engineering applications. Tissue Eng 141: 13-18.
- Chouhan D, Dey N, Bhardwaj N, Mandal BB (2023) Emerging and innovation. Cell J 25(3).
- Chavooshi R, Ranjkesh MR, Hashemi B, Roshangar L (2019) Cellulose and lignin-derived scaffold in tissue engineering vative approaches for wound healing and skin regeneration: current status and advances. Biomaterials 216: 119267.
- Fishman JA (2017) Infection in organ transplantation. Am J Transplant 17(4): 856-879.
- de Isla N, Huseltein C, Jessel N, Pinzano A, Decot V, et al. (2010) Introduction to tissue engineering and application for cartilage engineering. Biomed Mater Eng 20(3): 127-133.
- Stock UA, Vacanti JP (2001) Tissue engineering: current state and prospects. Annu Rev Med 52: 443-451.
- Ige OO, Umoru LE, Aribo S (2012) Natural products: a minefield of biomaterials. Int Sch Res Notices 983062.
- Karami MH, Abdouss M, Rahdar A, Pandey S (2024) Graphene quantum dots: background, synthesis methods, and applications as nanocarrier in drug delivery and cancer treatment: an updated review. Inorg Chem Commun 161: 112032.
- Zhang Y, Liu X, Zeng L, Zhang J, Zuo J, et al. (2019) Polymer fiber scaffolds for bone and cartilage tissue engineering. Adv Funct Mater 29(36): 1903279.
- Alzagameem A, Khaldi-Hansen BE, Kamm B, Schulze M (2018) Lignocellulosic biomass for energy, biofuels, biomaterials, and chemicals. Springer 95-132.
- Klemm D, Kramer F, Moritz S, Lindström T, Ankerfors M, et al. (2011) Nanocelluloses: a new family of nature-based materials. Angew Chem Int Ed Engl 50(24): 5438-5466.
- de Oliveira Barud HG, da Silva RR, da Silva Barud H, Tercjak A, Gutierrez J, et al. (2016) A multipurpose natural and renewable polymer in medical applications: bacterial cellulose. Carbohydr Polym 153: 406-420.
- Safari B, Aghanejad A, Kadkhoda J, Aghazade M, Roshangar L, et al. (2022) Biofunctional phosphorylated magnetic scaffold for bone tissue engineering. Colloids Surf B Biointerfaces 211: 112284.
- Nour S, Imani R, Chaudhry GR, Sharifi AM (2021) Skin wound healing assisted by angiogenic targeted tissue engineering: A comprehensive review of bioengineered approaches. J Biomed Mater Res A 109(4): 453-478.
- Bedian L, Villalba-Rodríguez AM, Hernández-Vargas G, Parra-Saldivar R, Iqbal HM (2017) Bio-based materials with novel characteristics for tissue engineering applications - A review. Int J Biol Macromol 98: 837-846.
- Behera SS, Das U, Kumar A, Bissoyi A, Singh AK (2017) Chitosan/TiO2 composite membrane improves proliferation and survival of L929 fibroblast cells: application in wound dressing and skin regeneration. Int J Biol Macromol 98: 329-340.
- Liu W, Du H, Zhang M, Liu K, Liu H, et al. (2020) Bacterial cellulose based composite scaffolds for biomedical applications: a review. ACS Sustainable Chem Eng 8(20): 7536-7562.
- Talikowska M, Fu X, Lisak G (2019) Application of conducting polymers to wound care and skin tissue engineering: a review. Biosens Bioelectron 135: 50-63.
- Machingal MA, Corona BT, Walters TJ, Kesireddy V, Koval CN, et al. (2011) A tissue-engineered muscle repair construct for functional restoration of an irrecoverable muscle injury in a murine model. Tissue Eng Part A 17(17-18): 2291-2303.
- Dvir T, Timko BP, Kohane DS, Langer R (2011) Nanotechnological strategies for engineering complex tissues. Nat Nanotechnol 6(1): 13-22.
- Jahangirian H, Lemraski EG, Rafiee-Moghaddam R, Webster TJ (2018) A review of using green chemistry methods for biomaterials in tissue engineering. Int J Nanomedicine 13: 5953-5969.
- Schmidt CE, Leach JB (2003) Neural tissue engineering: Strategies for repair and regeneration. Annu Rev Biomed Eng 5: 293-347.
- Diaz-Gomez L, Gonzalez-Prada I, Millan R, Da Silva Candal A, Bugallo-Casal A, et al. (2022) 3D printed carboxymethyl cellulose scaffolds for autologous growth factors delivery in wound healing. Carbohydr Polym 278: 118924.
- Eivazzadeh-Keihan R, Moghim Aliabadi HA, Radinekiyan F, Sobhani M, Khalili F, et al. (2021) Investigation of the biological activity, mechanical properties and wound healing application of a novel scaffold based on lignin-agarose hydrogel and silk fibroin embedded zinc chromite nanoparticles. RSC Adv 11(29): 17914-17923.
- Karami MH, Pourmadadi M, Abdouss M, Kalaee MR, Moradi O, et al. (2023) Novel chitosan/γ-alumina/ carbon quantum dot hydrogel nanocarrier for targeted drug delivery. Int J Biol Macromol 251:126280.
© 2024 Mohammad Hossein Karami and Majid Abdouss. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






