- Submissions

Full Text
Polymer Science: Peer Review Journal
A Quick Glance at Pickering Emulsions
Francisco López-Serrano1* and Benoit Fouconnier2
1Faculty of Chemistry, Department of Chemical Engineering, National Autonomous University of Mexico, Mexico
2Faculty of Chemical Sciences, Universidad Veracruzana, Mexico
*Corresponding author:Francisco López-Serrano, Faculty of Chemistry, Department of Chemical Engineering, National Autonomous University of Mexico, Mexico City 04510, Mexico
Submission: December 08, 2023;Published: January 25, 2024

ISSN: 2770-6613 Volume5 Issue3
Abstract
Given the multiple combinations that can be achieved by nanoparticles in an emulsifier-free environment, the use of such particles becomes important because surfactants may impact in a negative way the emulsion performance and its applications. The potential of hybrid particles for diverse applications, such as drug delivery, enzyme fixation, food, catalysis and photo-catalysis, coatings, agriculture and so forth, greatly motivates their study and understanding, both for research and industrial implementations.
Keywords:Pickering emulsions; Pickering stabilization
Introduction
An emulsion is a system in which two immiscible fluids coexist and one of them is dispersed throughout the other. This structure is not homogeneous; therefore, it is not a solution. Hence, the surface energy between two immiscible phases is generally very strong, which causes the system to be thermodynamically unstable. Thus, surface-active agents are required for the formation of this organization. In classical emulsions, soluble surfactants are used to stabilize emulsions. The findings that extremely fine solid water-insoluble particles could be used to stabilize oily droplets were first described by Ramsden [1]. Later, Pickering [2] performed research on emulsions and accomplished a more detailed understanding; thus, the term “Pickering stabilization” appeared. Work needs to be done on the system which consists of dispersing a liquid into another to form an emulsion [3]. Thus, this work is related to the product of the interfacial tension and the surface increase.
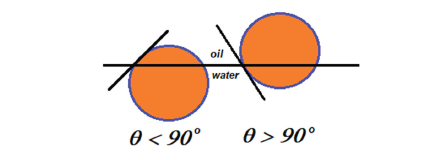
Schulman & Leja [4] reported that when the powder was more wettable by oil, this phase would be located in the emulsion continuous phase, and vice versa. Concerning the contact angle across the water phase at the solid-oil-water interfaces, if this angle is smaller than 90°, the solid particles are located at the oil-water interface, stabilizing a water-continuous emulsion (o/w). On the other hand, if the angle is greater than 90°, the particles are also held at the interface. However, now they stabilize an oil-continuous emulsion (w/o). This is presented in Figure 1. When the particles are totally wetted by the oil or by the water phases, they can be dispersed in either phase, respectively. In these cases, stable emulsions will not be formed.
Figure 1:The location of a spherical Pickering stabilizer, at a planar interface, depends on the contact angle.
Mini Review
Considering the case of a single layer stabilization by particles, if the contact angle q, is between 15° and 90°, o/w emulsions will be obtained. When the contact angle lies between 90° and 165°, then w/o emulsions will occur [5]. For small particles (neglecting gravity forces), Binks [6] proposed that the energy E required to remove solid particles with radius R from the interface, is given by equation (1).

The energy barrier (E) for the particle to re-enter (detach from the interface) the water phase is generally several orders of magnitude larger than the thermal energy. Thus, once the particles are adsorbed at the interface, detaching them will be very difficult. Therefore, this can be considered as an irreversible process. On the other hand, it is known that surfactants are considered to adsorb and desorb at relatively fast rates at the same time scale. When compared to traditional emulsions, this attribute makes Pickering emulsions considerably more stable. Other important facts that determine the stability of Pickering emulsions [7,8] are ratio of the oil phase to the continuous phase ratio, as well as each phase type. Other critical factors for determining stability are ionic strength and pH. The variations of pH change hydrophobicity; therefore, the solid particles wettability is also changed. A pH change modifies the ionic strength, altering the double layer electric structure and width. With reference to the techniques to determine morphology and interfacial characteristic properties of Pickering emulsions, the following methods have been reported: scanning electron microscopy, transmission electron microscopy, atomic force microscopy, bright field optical microscopy, phase contrast microscopy and confocal laser scanning. The reader is referred elsewhere [9] and references therein, for further information.
Regarding the preparation for obtaining Pickering emulsions,
the methods are ample and usually tailor-made. The readers
interested in this topic are referred elsewhere [10-12], and
references therein. In relation to Pickering emulsions applications,
the following examples have been cited: catalysis, photocatalysis,
antibacterial activity, filtration membranes, protein recognition
and drug delivery, among others [13]. At this point some definitions
are pertinent because some methods to manufacture Pickering
emulsions require polymerization:
(i) A Pickering emulsion is an emulsion (w/o or o/w or even
multiple) stabilized by solid particles [14-16].
(ii) A Pickering emulsion polymerization is an emulsion
polymerization process in which the colloidal stability is mainly
imparted by fine solid particles adsorbed at the interface of polymer
latex particles [17].
(iii) A Pickering mini emulsion or suspension polymerization
is a process whereby the polymerization takes place in emulsion
droplets already formed, stabilized by solid particles [18].
In the case of inorganic particles, Colard et al. [19] reported a pronounced effect on concentration, size and particle distribution of Pickering particles. Besides the key role that Pickering particles play in stabilizing the polymer particles, they also modify the polymerization rate and kinetics. In the case of inorganic particles. These authors also disclosed a pronounced effect on concentration, size and particle distribution of Pickering particles. A comprehensive work regarding polymerization of Pickering nanoemulsions via heterophase polymerization techniques in emulsion, mini emulsion, dispersion, and suspension has been presented by Shrade et al. [20]. In their work, they mention the preparation, characterization and applications using different types of particles such as: silica, laponite clay, magnetite, zinc oxide, titania and graphene oxide, among others.
The mechanistic events of a Pickering emulsion polymerization of methyl methacrylate and colloidal silica are detailed by Lotierzo & Bon [21]. These authors recorded that the adhesion of Pickering was not spontaneous, explaining that the particles are directed to the interface due to hetero coagulation, an event that occurs in the water phase of the growing oligo radicals. They also report pseudo-bulk kinetics, which occurs when several radicals coexist inside the polymerization particle, contrary to the 0-1 simple case [22]. Recently, it has been reported that in a styrene-silica Pickering emulsion polymerization, changing the pH and/or crosslinker (divinyl benzene) altered the hydrophobicity and Pickering emulsions structures and in specific conditions, percolated monoliths could be obtained [23,24].
Conclusion
After this brief glance at Pickering emulsions, their study offers boundless paths in the fields of science and industry.
Acknowledgment
FLS acknowledges DGAPA-PAPIIT Project IN112221, and FQUNAM PAIP 5000 9080 for financial support.
References
- Ramsden W (1904) Separation of solids in the surface-layers of solutions and ‘suspensions’ (observations on surface membranes, bubbles, emulsions, and mechanical coagulation). Proc R Soc London 72(477-486): 156-164.
- Pickering SU (1907) CXCVI.-emulsions. J Chem Soc Trans 91: 2001-2021.
- Finkle P, Draper HD, Hildebrand JH (1923) The theory of emulsification. J Am Chem Soc 45(12): 2780-2788.
- Schulman JH, Leja J (1954) Control of contact angles at the oil-water-solid interfaces emulsions stabilized by solid particles (BaSO4). Trans Faraday Soc 50: 598-605.
- Kaptay G (2006) On the equation of the maximum capillary pressure induced by solid particles to stabilize emulsions and foams and on the emulsion stability diagrams. Colloids Surfaces A: Physico Chem Eng Asp 387(401): 282-283.
- Zhang T, Liu F, Wu J, Ngai T (2022) Pickering emulsions stabilized by biocompatible particles: A review of preparation, bioapplication, and perspective. Particuology 64: 110-120.
- Binks BP, Lumsdon SO (2001) Pickering emulsions stabilized by monodisperse latex particles: effects of particle size. Langmuir 17(15): 4530-4547.
- Thickett SC, Zetterlund PB (2015) Graphene oxide (GO) nanosheets as oil-in-water emulsion stabilizers: influence of oil phase polarity. J Colloid Interface Sci 442: 67-74.
- Low LE, Siva SP, Ho YK, Chan ES, Tey BT (2020) Recent advances of characterization techniques for the formation, physical properties and stability of Pickering emulsion. Adv Colloid Interface Sci 277: 102117.
- Wu J, Ma GH (2016) Recent studies of Pickering emulsions: particles make the difference. Small 12(34): 4633-4648.
- Zhao H, Yang Y, Chen Y, Li J, Wang L, et al. (2022) A review of multiple Pickering emulsions: solid stabilization, preparation, particle effect, and application. Chem Eng Sci 248: 117085.
- Albert C, Beladjine M, Tsapis N, Fattal E, Agnely F, et al. (2019) Pickering emulsions: preparation processes, key parameters governing their properties and potential for pharmaceutical applications. J Control Release 309: 302-332.
- Gonzalez Ortiz D, Pochat-Bohatier C, Cambedouzou J, Bechelany M, Miele P (2020) Current trends in Pickering emulsions: particle morphology and applications. Engineering 6(4): 468-482.
- Aveyard R, Binks BP, Clint JH (2003) Emulsions stabilized solely by solid colloidal particles. Adv Colloid Interface Sci 100(102): 503-546.
- Binks BP (2002) Particles as surfactants-similarities and differences. Curr Opin Colloid Interface Sci 7(1-2): 21-41.
- Binks BP, Horozov TS (2006) Solids-Stabilized Emulsions: A Review. In: Binks BP, Horozov TS (Eds.), Colloidal Particles at Liquid Interfaces. Cambridge University Press, UK.
- Bon SAF (2015) Pickering emulsion polymerization. Encyclopedia of Polymeric Nanomaterials. pp. 1-6.
- Bon SAF (2014) Pickering suspension, mini-emulsion and emulsion polymerization. In: Ngai T, Bon S (Eds), Particle-stabilized emulsions and colloids: formation and applications. Cambridge: Royal Society of Chemistry, UK.
- Colard CAL, Teixeira RFA, Bon SAF (2010) Unraveling mechanistic events in solids-stabilized emulsion polymerization by monitoring the concentration of nanoparticles in the water phase. Langmuir 26(11): 7915-7921.
- Schrade A, Landfester K, Ziener U (2014) Pickering-type stabilized nanoparticles by heterophase polymerization. Chem Soc Rev 42: 6823-6839.
- Lotierzo A, Bon SAF (2017) A mechanistic investigation of Pickering emulsion polymerization. Polym Chem 8(34): 5100-5111.
- Gilbert RG (1995) Emulsion polymerization, a mechanistic approach. Academic Press, London.
- Fouconnier B, López Serrano F, Puente Lee RI, Terrazas RJE, Roman GA, et al. (2021) Hybrid microspheres and percolated monoliths synthesized via Pickering emulsion co-polymerization stabilized by in situ surface-modified silica nanoparticles. Express Polym Lett 15(6): 554-567.
- Fouconnier B, Aboudzadeh MA, López-Serrano F (2021) Silica-supported styrene-co-divinylbenzene Pickering emulsion polymerization: tuning surface charge and hydrophobicity by pH and co-aid adsorption. Processes 9(10): 1820.
© 2024 Francisco López-Serrano. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






