- Submissions

Full Text
Polymer Science: Peer Review Journal
Understanding the Effect of Unsaturation Content in Bromo-Butyl Rubber on Cure Kinetics
Tirthankar Bhandary, Hirak Satpathi*, Arnab Dutta, Sanjit Kumar Das, Saikat Dasgupta and Rabindra Mukhopadhyay
Hari Shankar Singhania Elastomer and Tyre Research Institute, India
*Corresponding author:Hirak Satpathi, Hari Shankar Singhania Elastomer and Tyre Research Institute, 437 Hebbal Industrial Area, Mysore-570016, Karnataka, India
Submission: August 22, 2023;Published: September 26, 2023

ISSN: 2770-6613 Volume5 Issue2
Abstract
Bromobutyl Rubber (BIIR) is increasingly used in elastomer industries for its unique physico-chemical and air barrier properties. This rubber is widely used in tyre Inner Liner (IL) compound due to very good air retention properties. In this study BIIR is mixed with Semi Reinforcing carbon black (SRF). Vulcanization characteristics and cure kinetics are studied on compound level. Due to low unsaturation in BIIR, Zinc Oxide (ZnO) is used in the vulcanization process as a curative. Here the study has been done with three BIIR having three different microstructure of different unsaturation content (1.77, 1.34 and 1.43) to see the effect in cure kinetics. At the end of this study it has been concluded that the activation energy in vulcanization reaction is decreasing with increase in degree of unsaturation.
Keywords:Bromobutyl rubber; Polymerisation; Vulcanization; Activation energy
Introduction
Bromobutyl Rubber (BIIR) is derived by bromination of butyl rubber acquired from cationic polymerisation of 1-2 mol% isoprene and isobutylene. Presence of bulky atom such as bromine helps BIIR to retain air. Due to its air retention property, BIIR is extensively used in tube and inner liner of bicycle, bike, truck, passenger car radial and industrial tyres. During vulcanization process that little unsaturation, 1-2mol%, present in the BIIR takes part in the vulcanization reaction. Vitiello R et al. [1], in their review article, has proposed three techniques to estimate unsaturation content in BIIR i.e., titration technique, infrared technique and nuclear magnetic resonance. Out of these three authors has endorsed NMR technique as a superlative technique to evaluate degree of unsaturation present in BIIR by dissolving a small portion of raw rubber in deuterated chloroform. Cheng et al. [2] proposed an equation (Equation 1) to estimate degree of unsaturation content in BIIR though NMR technique [2] and later R. Vitiello et all proposed alternative equations in their review article [1].
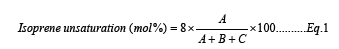
Where A, B and C are normalized integral signal intensity of region 5.0-5.1ppm, 4.9- 5.0ppm and 0.6-2.2 ppm respectively. Besides that Cao R et al. [3] has studied the impact of bromine content on cure rate and air permeability [3]. The author has concluded that the curing reactivity of the BIIR increases with increase in bromine content with an adverse effect on air permeability observed. In the present study, BIIR having three different unsaturation content have been analysed through NMR [1] and degree of unsaturation has been estimated using equation proposed by Cheng et al. [2]. Using BIIR samples comprising different degree of unsaturation has been mixed and effect of unsaturation on activation energy of vulcanization reaction through Arrhenius equation has been estimated.
Key Results and Discussion
A typical recipe for truck bus radial tyre inner liner has been selected. BIIR received from different supplier has been mixed following ISO 2393 in tangential mixer (Stewart bolling Banbury, volume 1.6L, Hudson, OH, USA) with 75% of fill factor in two different stages. In this study the author has applied the equation suggested by Vitiello R et al. [1] for estimating the residual unsaturation content in different BIIR samples through 1H NMR technique. The unsaturation content is found to be 1.77, 1.34 and 1.43mol% for BIIR-A, BIIR-B and BIIR-C respectively (calculated using Equation 1). From the DSC study of the compounds, it has been observed that the curing enthalpy of Comp-A, Comp-B and Comp-C is 4.921J/g, 3.591J/g and 4.066J/g respectively. A correlation between degree of unsaturation present in raw BIIR samples and enthalpy required in vulcanization reaction has been drawn and it is observed that the enthalpy of vulcanization increases with increase in degree of unsaturation (in a linear relationship with regression coefficient faction of 0.974). Further the effect of degree of unsaturation on vulcanization kinetics of BIIR has been evaluated by estimating the vulcanization rate, K, obtained using Equation 2 and Arrhenius Equation 3.
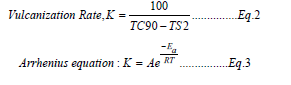
Where K is the rate constant of vulcanization reaction, A is pre-exponential factor, Ea is the activation energy required for vulcanization reaction, R is universal gas constant and T is the temperature in absolute unit. To estimate the activation energy required in vulcanization reaction of different compounds, the Equation 4, derived from Arrhenius equation has been used.
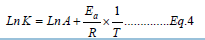
A correlation curve between Ln K and 1/T has been drawn for Comp-A, Comp-B and Comp-C. From the intercept, A and from the slope, Ea of the compound has been evaluated. A relation between degree of unsaturation in the raw rubber and activation energy has been drawn and found that activation energy in vulcanization reaction is decreasing with increase in degree of unsaturation.
References
- Vitiello R, Tesser R, Turco R, Santacesaria E, Compagnone G, et al. (2017) A critical review on analytical methods and characterization of butyl and bromobutyl rubber. Int J Polym Anal Charact 22(4): 348-360.
- Cheng DM, Gardener IJ, Wang HC, Frederick CB, Dekmezian AH, et al. (1990) Spectroscopic studies of the structures of butyl and bromobutyl rubbers. Rubber Chemistry and Technology 63(2): 265-275.
- Cao R, Zhao X, Zhao X, Wu X, Li X, et al. (2019) Bromination modification of butyl rubber and its structure, properties, and application. Ind Eng Chem Res 58(36): 16645-16653.
© 2023 Hirak Satpathi. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






