- Submissions

Full Text
Polymer Science: Peer Review Journal
Chitosan Based Layers for Biomedical and Environmental Applications
Didem Demir1*, Sabri Kalkan2 and Seda Ceylan3
1Department of Chemistry and Chemical Process Technologies, Tarsus University, Türkiye
2Department of Occupational Health and Safety, Toros University, Türkiye
3Department of Bioengineering, Adana Alparslan Turkes Science and Technology University, Türkiye
*Corresponding author:Didem Demir, Department of Chemistry and Chemical Process Technologies, Tarsus University, Mersin Tarsus Organized Industrial Zone, Akdeniz, Mersin, Türkiye
Submission: August 16, 2023;Published: September 05, 2023

ISSN: 2770-6613 Volume5 Issue2
Abstract
As a natural, biocompatible and biodegradable polymer, chitosan is used for many different applications. Other important features of chitosan are its environmental friendliness, its renewable resource and its inherent high antimicrobial activity. It can be used directly or composites with other polymers in different forms such as spherical, layer, monolithic, particles, etc. Among these, chitosan coatings are applied in the form of thin films and membranes by methods such as solvent casting, electrospinning, dip coating and spin coating. Chitosan films are used in active packaging for the food industry, wound dressings, drug delivery systems and scaffolds for biomedical applications, water treatment and corrosion prevention for environmental applications. In this review, we focus on chitosan-based films, membranes and coatings developed specifically for biomedical and environmental applications. Firstly, information about chitin, chitosan and chitosan derivatives, functional properties of chitosan are presented. Subsequently, the preferred methods for the production of chitosan-based films were reviewed. In the last part, studies on chitosan films for environmental and biomedical applications and current status assessment were presented.
Keywords:Biopolymer; Chitosan; Membrane; Film; Biomedicine; Environment
Abbreviations:BCP: Biphasic Calcium Phosphate; CDHA: Hydroxyapatite; CS: Chitosan; DD: Degree of Deacetylation; EGF: Epidermal Growth Factor; PCL: Poly (Caprolactone); PEO: Poly (Ethylene Oxide); PVA: Poly (Vinyl Alcohol); St: Starch; RI-Modified Chitosan: Resin I Modified Chitosan; RII-Modified Chitosan: Resin II Modified Chitosan; TCP: β‑Tricalcium Phosphate; ZnO: Zinc Oxide
Introduction
Chitosan is a biopolymer obtained by processing the second most abundant polysaccharide in nature, chitin, present in green algae, the cell walls or fungi and in the exoskeleton of crustaceans [1,2]. Different fabrication techniques have been developed to convert chitosan polymer into a usable and applicable material. It is suitable to be produced in many forms, either alone or in combination with other polymers and/or additives. For different areas of applications, it can be used in the form of solutions, suspensions, hydro/ cryo/aerogels, powders, micro/nanoparticles, beads, sponges, foams, membranes and films or micro/nanofibers [3]. Thanks to its unique properties, chitosan, which has the potential to be used for many different application areas (medicine, food, pharmaceuticals, cosmetics, agriculture, textiles, pulp and paper, and environmental chemistry), is inherently biodegradable, biocompatible and antibacterial [4-7]. The two different area that attracts attention among these fields covers chitosan-based materials produced for biomedicine and environment. Environmentally, it can be used for remediation of contaminated soil and wastewater, while for biomedical applications it can be used for tissue regeneration, wound dressing material, drug delivery systems, and cosmetic purposes. When the studies carried out so far are examined, it is seen that the easiest way to evaluate chitosan and to demonstrate its effectiveness for the aforementioned applications is through the preparation of chitosan-based thin films. Among the common synonyms of the word “film” mentioned here are the keywords membrane, barrier, layer and coating. Therefore, these synonyms may also be encountered in the remainder of the review. Based on these definitions, the review focuses on the evaluation of films produced using chitosan natural polymer for biomedical and environmental applications. In the studies carried out so far, we see that a large number of reviews have been prepared on the materials produced using chitosan and its derivatives. For example, Cosmetic [8], Biomaterial [9], Food Packaging [10], Electrochemical Sensors [11] and adsorption of pollutants [12], etc.
However, chitosan has been studied by many researchers for many applications in different forms with different production techniques. It will be more effective and understandable to narrow and customize this broad perspective and to focus on specific application areas as chitosan films in this review for a chosen form. Therefore, in this study, as an innovative side, we wanted to evaluate chitosan-based membranes/films for biomedical and environmental applications, which are two important application areas. In this context, first the definition and properties of chitosan, then the preferred fabrication methodologies for the production of chitosan-based films, and finally the evaluation of these materials for biomedical and environmental applications, which are two different fields as well as having many applications.
Chitosan and its Structure
Chitin is a naturally occurring mucopolysaccharide exist in the exoskeleton and cell walls of crustaceans, insects, and fungi. It is one of the most abundant polysaccharides on the planet, ranking second only to cellulose. Chitin is made up of a sugar backbone with β-1,4- linked glucosamine units and a high level of acetylation. Due to its important properties, which include nontoxicity, biocompatibility, and biodegradability, the ability to be degraded by enzymes, chitin has recently attracted a great deal of attention from scientists, allowing it to be used in numerous biomedical applications, such as drug delivery, tissue engineering, wound healing, and cancer therapy [13]. The most important chitin derivative is chitosan, which is typically produced by alkaline deacetylation of chitin. Due to its low toxicity and biodegradability, chitosan has found widespread use in the pharmaceutical industry [14,15]. In terms of other properties, chitosan is a white, odourless powder (or granules) with varying molecular weight, Degree of Deacetylation (DD), solubility in diluted hydrochloric, formic, and acetic acids, and insolubility in water and organic solvents. The melting point is roughly 290 °C [16,17]. Thus, the dissolution of chitosan in dilute hydrochloric acid is due to the interaction of amino groups with hydrogen cations, which converts it into a polyelectrolyte with a positive charge [18,19]. For chemical aspect, chitosan is a natural linear polysaccharide composed of arbitrarily distributed β-(1-4)-linked D-glucosamine and N-acetyl-D-glucosamine, i.e. it is made up of two monosaccharide units: D-glucosamine and N-acetyl-D-glucosamine. In 2-amino-2-deoxy-β-D-glucopyranose and 2-acetamido-2-deoxy-β-D-glucopyranose linked by β-(1–4) glycosidic bonds, approximately fifty percent of the acetyl groups will be removed from the chitin via hydration or enzyme hydrolysis [20,21]. When the DD is greater than 50%, the polymer is referred to as chitosan; otherwise, it is referred to as chitin [22,23]. In the process, sodium borohydride is added, or the system is purged with nitrogen to prevent depolymerization and the formation of reactive particles under the influence of oxygen [24]. Also, in different research, scientists discovered the chitosan has α, β and γ crystal structures [25]. The α-chitosan (principal form) has a compact structure with strong intermolecular interactions and is composed of two polysaccharide chains arranged in an inverse manner. The β-chitosan consists of two aligned, parallel polysaccharide chains with weak intermolecular hydrogen bonds. The γ-chitosan consists of three parallel polysaccharide chains, two of which are aligned in the same direction and one in the opposite direction. The α-chitosan is derived from crustaceans and prawns, β-chitosan from squids, and γ-chitosan from loligos [24-27].
The Functional Properties of Chitosan
Chitosan, a natural biopolymer, is biodegradable, non-toxic and non-allergenic and is therefore in increasing demand as a natural resource. It is a safe polymer approved by the Food and Drug Administration. Due to its inherent biological properties such as antioxidant, antibacterial, antivirus, antifungal, anticancer, immunomodulatory, wound healing and hemostatic, it is a very popular resource especially for the pharmaceutical industry, biomedical applications and food industry [28]. In addition, due to its easy availability, good adsorption capacity, biocompatibility, and nonecotoxicity, it is an effective source for the purification of water and wastewater from heavy metals, dyes, microorganisms, salts and other contaminants such as fungi, radionuclides and fluoride [29]. However, poor mechanical properties and low solubility limit the use of chitosan. Therefore, its use either by blending with other polymers such as poly (vinyl alcohol), poly (methyl methacrylate), polyaniline, polysulfone, polycarbonate, gelatin, pectin and cellulose or by adding nanoparticles/actives such as Silver, Iron, Zinc Oxide, Cobalt, Hydroxyapatite Nanoparticles And Plant Extracts, Drugs, Essential Oils is attracting attention [30].
Methods for Chitosan-Based Membrane Production
When the production methods of chitosan-based layers, which can also be defined as membrane, coating, film or layer, are examined, it is seen that solvent casting [31,32], compression molding [33], surface coating [34], electrospinning [35,36], spin coating [37] and low-pressure low-temperature nanoimprinting [38] methods are used. In almost all of the aforementioned methods, chitosan must first be dissolved in a suitable solvent and become a solution. In general, chitosan is soluble in acid solutions such as hydrochloric acid and in many organic acids, including formic, acetic, butyric, malic, citric, lactic, oxalic, propionic, and succinic acids, at varying concentrations. However, in studies carried out so far, the most commonly used solvent to dissolve chitosan of different molecular weights is acetic acid [2,39-41].
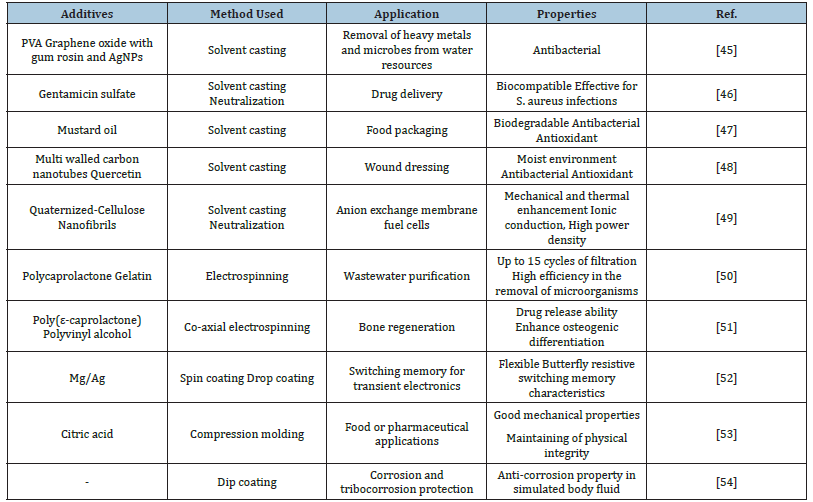
Among the methods, solvent casting, also known as solution casting, is probably the easiest and least expensive method of fabricating chitosan-based films. The basis of this technique is to pour a homogeneously prepared solution of chitosan in a suitable solvent into a mold and then allow the solvent to evaporate to leave a solid chitosan film that can be peeled off the mold. As another popular method, electrospinning, is one of the most effective technologies for preparation of nano/micro fibrous membranes [42-44]. The method is based on the formation of fibers in the collector plate by stretching the chitosan polymer in jet form under the electrical field created by a high voltage power source. The accumulated fibers are peeled from the collecting plate and used as a membrane. Using conventional and modified electrospinning setup for fiber production, it is possible to produce random, aligned, hollow and core-shell fiber structures. In Table 1, besides solvent casting and electrospinning as the most commonly used methods, the preferred methods for the production of chitosan-based sheets, the additives used, the application area and the properties gained for the area of interest are presented by reviewing the current studies.
Table 1:Methods used in the production of layers based on chitosan and its composites, application area and features gained for the field of interest.
Applications
Biomedical applications
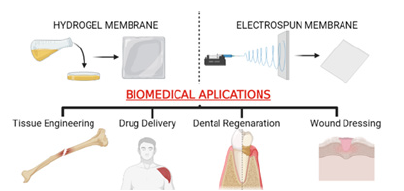
Chitosan based membrane systems have been extensively utilized as potential biomedical applications. Some important applications of chitosan-based delivery devices in the pharmaceutical industry are discussed in this section. In addition, this section discusses the biomedical applications and biological characteristics of chitosan-based scaffolds as thin membranes in tissue engineering applications, such as wound dressing, dental regeneration and bone tissue engineering [45-50]. The application areas of chitosan based thin membranes produced by electrospinning and solvent casting, one of the most preferred production methods within the scope of biomedical applications, are summarized in Figure 1.
Figure 1:Biomedical applications in tissue engineering such as drug delivery, dental regeneration, bone tissue engineering and wound dressing of chitosan based thin membranes produced in hydrogel and fibrous structure.
Electrospinning, thermally induced phase separation, and selfassembly can be used to create the membrane scaffolds, which are extensively used in biomedical applications such as biological scaffolds, drug delivery, bacterial inhibition, and wound dressing. Recently, it was discovered that certain chitin/chitosan-based nanofibrous scaffolds share structural similarities with bone’s extracellular matrix and can aid in bone regeneration [51-54]. In one research article, using the layer-by-layer method, chitosan and the cytokines BMP2 and BMP7 can be deposited onto Poly (Caprolactone) (PCL) nanofibrous scaffolds. Both chitosan and cytokines can function synergistically to promote osteoblast development in vitro and facilitate bone healing in vivo [55,56]. Calcium Phosphates and Chitosan (CS) have been composed of for bone tissue engineering and evaluated in different research article. Three different calcium phosphates were synthesized: Calcium Deficient Hydroxyapatite (CDHA), Biphasic Calcium Phosphate (BCP) and β‑Tricalcium Phosphate (TCP) by a reverse emulsion method followed by calcination and compared their efficacy on bone regeneration. Histological and histomorphometric analyses demonstrated that the BCP/CS membrane promoted the most efficient bone regeneration when compared to the other two hybrid membranes. At three weeks post-surgery, the BCP/CS membrane could increase the formation of new bone by up to 57% of the initial bone defect area.
Therefore, the BCP/CS membrane has the potential to be used for guided bone regeneration [57]. For drug release applications, to increase the hydrophobicity of the membranes and protect their nanofibrous structure, chitosan membranes were modified with acylation reactions involving fatty acids with varying chain lengths or tert-butyloxycarbonyl protecting groups. These membranes were then examined to control the release of a hydrophobic osteogenic drug-Simvastatin (SMV). The membranes composed of long chain fatty acids released SMV for up to 90 days. Molecular modelling corroborated these findings by revealing that SMV was more compatible with Hydrophobic Membranes [58]. Dental tissue engineering also use chitosan membranes for regeneration. In a research study, the goal was developing experimental composite polymers with chitosan or chitosan loaded with Dibasic Calcium Phosphate Anhydrous (DCPA) particles and to demonstrate their antimicrobial potential without sacrificing mechanical properties or biocompatibility. Results showed that, no chitosan release was detected from the composites, suggesting that the observed antimicrobial effect was due to direct contact between bacteria and surface-exposed chitosan particles. The addition of chitosan or chitosan/DCPA particulates to restorative composites resulted in antimicrobial activity without compromising the composites’ mechanical properties or biocompatibility [59].
Independently, essential oils of lemon balm (Melissa officinalis L.) and dill (Anethum graveolens L.) were encapsulated into collagen hydrolysates extracted from bovine tendons and rabbit skins, both mixed with chitosan, for potential wound dressing applications. The synergistic effect of dill and lemon balm essential oils can enhance the antibacterial activity of collagen hydrolysate-chitosan nanofibers against the most prevalent bacterial strains. The in vivo test results indicated that electrospun samples consisting of collagen hydrolysate extracted from bovine tendons or rabbit skin, combined with chitosan, and containing dill and/or lemon balm essential oils as encapsulated bioactive compounds were biocompatible [60]. In another research, nonwoven scaffolds incorporate growth factors such as vascular endothelial growth factor, Epidermal Growth Factor (EGF), and transforming growth factor beta 1. As suggested by Nejaddehbashi et al. [61] growth factors related to connective tissue can also be incorporated into the nonwoven scaffold for use as a wound dressing. The authors created a three-layer scaffold with a CS/PEO electrospun nonwoven in the middle. During the electrospinning process, EGF and basic fibroblast growth factor were incorporated and increased cell proliferation, thereby accelerating angiogenesis and wound healing [61]. Antimicrobial activity is also another aim for wound dressing or tissue engineering applications. For this reason, hydrogel membranes were fabricated based on Poly Vinyl Alcohol (PVA), Starch (St), and Chitosan (CS) hydrogels with nano Zinc Oxide (ZnO). The examination revealed that hydrogel membranes were more effective as a wound dressing in the early phases of wound recovery and that the gel could be used in topical applications requiring a broad spectrum of antibacterial activity, specifically as a wound dressing bandage [62]. The same goal was targeted for non-healing wound as serious complication of diabetes, in this study, researchers hypothesised that nanofiber mats composed of chitosan, Polyvinyl Alcohol (PVA), and ZnO could be an effective option for accelerating the healing of diabetic wounds due to the wound healing activities of chitosan-PVA nanofibers and the antibacterial properties of ZnO. Compared to chitosan/ PVA nanofibrous membranes, chitosan/PVA/ZnO nanofibrous membranes exhibited greater antibacterial activity against E. coli, P. aeruginosa, B. subtilis, and S. aureus. In addition, chitosan/PVA/ ZnO nanofibrous membranes had a greater antioxidant capacity than chitosan/PVA nanofibrous mats. Compared to chitosan/PVA nanofibers, chitosan/PVA/ZnO nanofibrous membranes resulted in accelerated wound healing in in vivo wound healing investigations. Therefore, the present investigation demonstrates that chitosan/ PVA/ZnO electrospun scaffolds may be useful as dressings for diabetic wounds [63].
Environmental applications
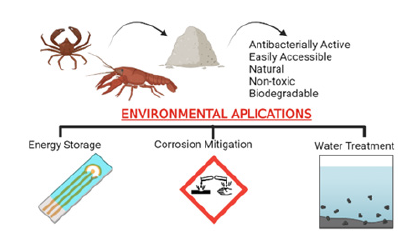
Emerging contaminant concentrations are widely found in numerous industrial commodities extensively used by society, encompassing pharmaceuticals, personal care products, and pesticides. The elevated presence of emerging contaminants within domestic and industrial wastewater has evolved into a significant concern for public health in contemporary times. Consequently, a multitude of techniques and materials are employed within wastewater treatment processes to address the removal of these pollutants. Due to its easily accessible nature, non-toxic effects, and environmental harmlessness, chitosan is widely employed in sectors such as food and beverages, cosmetics and pharmaceuticals [64]. Its eco-friendliness and facile natural degradation have prompted researchers to investigate the effects of chitosan on pollutant removal and environmental applications [65]. The most common environmental applications where chitosan has the potential to be used, thanks to its important functional properties, are shown in the Figure 2. A few examples of the environmental applications of chitosan and modified chitosan are outlined as follows: Electricity generation is a realm where efforts to curtail human ecological impact have steered the trajectory towards the augmented utilization of renewable sources such as solar, wind, and hydroelectric systems? The integration of renewable resources into the fabric of electricity production assumes paramount importance in the context of fostering a sustainable societal milieu. Notwithstanding the progress, it remains conspicuous that prevailing paradigms governing the design and composition of rechargeable batteries, the ubiquitous energy reservoirs of contemporary society, are still remote from a comprehensive alignment with environmentally conscientious aspirations. In particular, the incumbently standardized technologies underpinning lithium-ion batteries notably belie ecological sensitivity. The regular recourse to cobalt as a preferred cathode material for lithium-ion batteries encapsulates a conundrum, given its scarcity, financial exigency, and the propensity to engender hazardous waste within the recycling loop. In this purview, the proposition of cathode alternatives emerges as a focal point. Within this discourse, the juxtaposition of chitosan and lignin as prospective candidates assumes particular significance. The allure of these materials stems from their innate abundance within the natural milieu, concomitant with their benign ecological profiles. A pertinent illustration is provided by the work of Ilıc et al. [66], wherein the augmentation of chitosan electrodes with vanillin engenders a cathode system that seamlessly accommodates energy storage up to a noteworthy 80mA hg-1 [66].
Figure 2:Environmental applications of chitosan coating as a layer with its important functional properties.
Corrosion, a matter of significant global apprehension, engenders considerable economic and environmental losses by rendering functional materials non-functional. The primary strategy employed for corrosion mitigation entails the utilization of corrosion inhibitors applied onto surfaces vulnerable to corrosion. These inhibitors encompass structures containing N, O and S bonds that possess the capacity to engage their unpaired electrons with the metal surface. Alternatively, they can manifest as polymer materials forming a protective coating on the metal surface. Natural polymers, exemplified by chitosan, exhibit compatibility with the ecosystem, abundant presence in nature, and cost-effectiveness. They establish interaction with metals via the -NH2 functional groups embedded within their molecular chains, thereby furnishing protection against corrosion [67]. Silver, naturally occurring in compound forms within the Earth’s crust, is utilized in various sectors due to its ease of processing, high electrical and thermal conductivity, as well as its antimicrobial properties. These sectors include battery production, medical and dental applications, and water purification systems. However, the presence of dissolved silver ions in water can be hazardous to human health. Therefore, the removal of dissolved silver ions from water is crucial. This removal process can be achieved through various methods, including chemical precipitation, ion exchange, solvent extraction, and using materials with high adsorption capacity like chitosanbased substances. In their study conducted in 2021, El-shorbagy et al. [68] successfully achieved the removal of toxic silver ions from water using adsorption with chitosan modified with RI and RII additives. In the presence of chitosan, they obtained 22.5% silver removal, 100% with RI-modified chitosan, and 92.6% with RIImodified chitosan [68].
Cardoso & Vitali [69] conducted an investigation into the adsorption-mediated removal of isoniazid, cortisol, bisphenol A, 17α-ethinylestradiol, and triclosan-compounds acknowledged for their adverse effects on human health-utilizing chitosan and vanillin-modified chitosan. The study entailed an examination of the removal of these substances from aqueous solutions across the pH spectrum ranging from 5 to 10. Their findings revealed that the peak removal efficiency for chitosan was achieved at pH 9, whereas for vanillin-modified chitosan, the maximal removal efficiency was observed at pH 8. Additionally, it was observed that 17α-ethinylestradiol exhibited the highest removal efficiency for both adsorbents [69].
Conclusion
This review was prepared with the aim of revealing the potential and current situation of chitosan films in biomedical and environmental protection applications. Studies show that the antimicrobial, biocompatible and biodegradable properties of chitosan are mainly utilized. In addition, due to its low mechanical properties and low solubility, it is generally used together with other synthetic polymers such as PVA, PCL and PEO. In addition, recent studies continue to use metal nanoparticles combined with essential oils and plant extracts to add functional properties to chitosan films. In biomedical applications, chitosan has been studied in a wide area due to its high biocompatibility, non-toxicity, degradability, antimicrobial and antioxidant properties. However, when we look at the environmental applications, it can be said that this area is a more virgin area for chitosan-based films/coatings/ layers. For wastewater treatment, attempts can be made to remove antibiotics, heavy metals and microbial contaminants due to its high adsorption potential towards a wide range of contaminants such as organic compounds, dyes and heavy metals through ionexchange or electrostatic attraction. For this reason, there is a need to develop the physicochemical properties and biological activities of chitosan-based films developed especially for environmental applications in more detail.
References
- Antonino RSCMDQ, Fook BRPL, Lima VADO, Rached RÍDF, Lima EPN, et al. (2017) Preparation and characterization of chitosan obtained from shells of shrimp (Litopenaeus vannamei boone). Mar Drugs 15(5): 141.
- Demir D, Öfkeli F, Ceylan S, Bölgen N (2016) Extraction and characterization of chitin and chitosan from blue crab and synthesis of chitosan cryogel scaffolds. J Turkish Chem Soc Sect A Chem 3(3): 131-144.
- Morin-Crini N, Lichtfouse E, Torri G, Crini G (2019) Fundamentals and applications of chitosan. Sustain Agric Rev 35: 49-123.
- Elieh-Ali-Komi D, Hamblin MR, Daniel EAK (2016) Chitin and chitosan: production and application of versatile biomedical nanomaterials. Int J Adv Res 4(3): 411-427.
- Kabalak M, Aracagök D, Torun M (2020) Extraction, characterization and comparison of chitins from large bodied four Coleoptera and Orthoptera Int J Biol Macromol 145: 402-409.
- Panariello L, Vannozzi A, Morganti P, Coltelli MB, Lazzeri A (2021) Biobased and eco-compatible beauty films coated with chitin nanofibrils, nanolignin and vitamin E. Cosmet 8(2): 27.
- Morin-Crini N, Lichtfouse E, Torri G, Crini G (2019) Applications of chitosan in food, pharmaceuticals, medicine, cosmetics, agriculture, textiles, pulp and paper, biotechnology, and environmental chemistry. Environ Chem Lett 17(4): 1667-1692.
- Kulka K, Sionkowska A (2023) Chitosan based materials in cosmetic applications: A review. Molecules 28(4): 1817.
- Victor R de S, Santos AM da C, de Sousa BV, Neves G de A, Santana LN de L, et al. (2020) A review on chitosan’s uses as biomaterial: tissue engineering, drug delivery systems and cancer treatment. Materials 13(21): 4995.
- Flórez M, Guerra-Rodríguez E, Cazón P, Vázquez M (2022) Chitosan for food packaging: Recent advances in active and intelligent films. Food Hydrocoll 124: 107328.
- Annu, Raja AN (2020) Recent development in chitosan-based electrochemical sensors and its sensing application. Int J Biol Macromol 164: 4231-4244.
- Saheed IO, Oh W Da, Suah FBM (2021) Chitosan modifications for adsorption of pollutants - A review. J Hazard Mater 408: 124889.
- Baharlouei P, Rahman A (2022) Chitin and chitosan: Prospective biomedical applications in drug delivery, cancer treatment, and wound healing. Mar Drugs 20(7): 460.
- Roberts GAF (1992) Structure of chitin and chitosan. Chitin Chem, Palgrave, London, pp. 1-53.
- Ahsan SM, Thomas M, Reddy KK, Sooraparaju SG, Asthana A, et al. (2018) Chitosan as biomaterial in drug delivery and tissue engineering. Int J Biol Macromol 110: 97-109.
- Sivaramakrishna D, Bhuvanachandra B, Mallakuntla MK, Das SN, Ramakrishna B, et al. (2020) Pretreatment with KOH and KOH-urea enhanced hydrolysis of α-chitin by an endo-chitinase from Enterobacter cloacae cloacae. Carbohydr Polym 235: 115952.
- Goy RC, Britto D De, Assis OBG (2009) A review of the antimicrobial activity of chitosan. Polim Cienc e Tecnol 19(3): 241-247.
- Younes I, Rinaudo M (2015) Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar Drugs 13(3): 1133-1174.
- Islam S, Bhuiyan MAR, Islam MN (2017) Chitin and chitosan: structure, properties and applications in biomedical engineering. J Polym Environ 25: 854-866.
- Zhao Y, Ma L, Zeng R, Tu M, Zhao J (2016) Preparation, characterization and protein sorption of photo-crosslinked cell membrane-mimicking chitosan-based hydrogels. Carbohydr Polym 151: 237-244.
- Pereira L, Alves M (2012) Dyes-environmental impact and remediation. Environ Prot Strateg Sustain Dev pp. 111-162.
- Bajaj M, Winter J, Gallert C (2011) Effect of deproteination and deacetylation conditions on viscosity of chitin and chitosan extracted from Crangon crangon shrimp waste. Biochem Eng J 56(1-2): 51-62.
- Yong SK, Shrivastava M, Srivastava P, Kunhikrishnan A, Bolan N (2015) Environmental applications of chitosan and its derivatives. Rev Environ Contam Toxicol 233: 1-43.
- Jollès P, Muzzarelli RAA (1999) Chitin and chitinases. Springer, 702.
- Jang M, Kong B, Jeong Y, Lee CH, Nah J (2004) Physicochemical characterization of α‐chitin, β‐chitin, and γ‐chitin separated from natural resources. J Polym Sci Part A Polym Chem 42(14): 3423-3432.
- Anitha A, Sowmya S, Kumar PTS, Deepthi S, Chennazhi KP, et al. (2014) Chitin and chitosan in selected biomedical applications. Prog Polym Sci 39(9): 1644-1667.
- Kaya M, Mujtaba M, Ehrlich H, Salaberria AM, Baran T, et al. (2017) On chemistry of γ-chitin. Carbohydr Polym 176: 177-186.
- Hafsa J, Smach MA, Mrid R Ben, Sobeh M, Majdoub H, et al. (2021) Functional properties of chitosan derivatives obtained through Maillard reaction: A novel promising food preservative. Food Chem 349: 129072.
- Zubair M, Arshad M, Ullah A (2020) Chitosan-based materials for water and wastewater treatment. Handb Chitin Chitosan Chitin- Chitosan-based Polym Mater Var Appl 3: 773-809.
- Croisier F, Jérôme C (2013) Chitosan-based biomaterials for tissue engineering. Eur Polym J 49(4): 780-792.
- Kumar H, Ahuja A, Kadam AA, Rastogi VK, Negi YS (2022) Antioxidant film based on chitosan and tulsi essential oil for food packaging. Food Bioprocess Technol 16: 342-355.
- Lieder R, Darai M, Örlygsson G, Sigurjonsson OE (2013) Solution casting of chitosan membranes for in vitro evaluation of bioactivity. Biol Proced Online 15(1): 11.
- Infurna G, Cavallaro G, Lazzara G, Milioto S, Dintcheva NT (2022) Effect of different processing techniques and presence of antioxidant on the chitosan film performance. J Vinyl Addit Technol 28(2): 343-351.
- Oberlintner A, Vesel A, Naumoska K, Likozar B, Novak U (2022) Permanent hydrophobic coating of chitosan/cellulose nanocrystals composite film by cold plasma processing. Appl Surf Sci 597: 153562.
- Avila LB, Pinto D, Silva LFO, de Farias BS, Moraes CC, et al. (2022) Antimicrobial bilayer film based on chitosan/electrospun zein fiber loaded with jaboticaba peel extract for food packaging applications. Polym 14(24): 5457.
- Gulzar S, Tagrida M, Nilsuwan K, Prodpran T, Benjakul S (2022) Electrospinning of gelatin/chitosan nanofibers incorporated with tannic acid and chitooligosaccharides on polylactic acid film: Characteristics and bioactivities. Food Hydrocoll 133: 107916.
- Ligler FS, Lingerfelt BM, Price RP, Schoen PE (2001) Development of uniform chitosan thin-film layers on silicon chips. Langmuir 17(16): 5082-5084.
- De Masi A, Tonazzini I, Masciullo C, Mezzena R, Chiellini F, et al. (2019) Chitosan films for regenerative medicine: fabrication methods and mechanical characterization of nanostructured chitosan films. Biophys Rev 11(5): 807-815.
- Qiao C, Ma X, Wang X, Liu L (2021) Structure and properties of chitosan films: Effect of the type of solvent acid. LWT 135: 109984.
- Demir D, Bölgen N (2017) Synthesis and characterization of injectable chitosan cryogel microsphere scaffolds. Int J Polym Mater Polym Biomater 66(13): 686-696.
- Öfkeli F, Demir D, Bölgen N (2020) Biomimetic mineralization of chitosan/gelatin cryogels and in vivo biocompatibility assessments for bone tissue engineering. J Appl Polym Sci 138(14): 50337.
- Khalili S, Ghane N, Khorasani SN, Heydari F, Atwal A, et al. (2022) Cytocompatibility and antibacterial properties of coaxial electrospun nanofibers containing ciprofloxacin and indomethacin drugs. Polym 14(13): 2565.
- Demir D, Güreş D, Tecim T, Genç R, Bölgen N (2018) Magnetic nanoparticle-loaded electrospun poly(ε-caprolactone) nanofibers for drug delivery applications. Appl Nanosci 8: 1461-1469.
- Saleh M, Demir D, Ozay Y, Yalvac M, Bolgen N, et al. (2021) Fabrication of basalt embedded composite fiber membrane using electrospinning method and response surface methodology. J Appl Polym Sci 138(25): 50599.
- Ahmed EM, Isawi H, Morsy M, Hemida MH, Moustafa H (2023) Effective nanomembranes from chitosan/PVA blend decorated graphene oxide with gum rosin and silver nanoparticles for removal of heavy metals and microbes from water resources. Surfaces and Interfaces 39: 102980.
- Supernak M, Makurat-Kasprolewicz B, Kaczmarek-Szczepá nska B, Pałubicka A, Sakowicz-Burkiewicz M, et al. (2023) Chitosan-based membranes as gentamicin carriers for biomedical applications--influence of chitosan molecular weight. Membr 13(6): 542.
- Mohan S, Unnikrishnan TG, Dubey U, Ramesh M, Panneerselvam K (2023) Development and characterization of mustard oil incorporated biodegradable chitosan films for active food packaging applications. J Polym Environ 31: 2190-2203.
- Baktehir NH, Arbi MAM, Selvaras T, Ismail NI (2023) Blend of multi-walled carbon nanotubes and quercetin improves physicochemical properties of chitosan membrane for wound dressing application. Malaysian J Fundam Appl 19(2): 202-214.
- Hren M, Makuc D, Plavec J, Roschger M, Hacker V, et al. (2023) Efficiency of neat and quaternized-cellulose nanofibril fillers in chitosan membranes for direct ethanol fuel cells. Polym 15(5): 1146.
- Baburaj MS, Veeran MG, Painuly D, Sreelekshmi S, Rajkumar RJ, et al. (2023) Fabrication and characterisation of polycaprolactone/gelatin/chitosan (PCL/GEL/CHI) electrospun nano-membranes for wastewater purification. Desalination 563: 116709.
- Zhu Y, Jiang S, Xu D, Cheng G, Shi B (2023) Resveratrol-loaded co-axial electrospun poly(ε-caprolactone)/chitosan/polyvinyl alcohol membranes for promotion of cells osteogenesis and bone regeneration. Int J Biol Macromol 249: 126085.
- He N, Sun Y, Yuan Q, Wang Y, Zuo S (2023) Biodegradable natural chitosan coating films-based flexible resistive switching memory for transient electronics. Mater Sci Eng B 295: 116578.
- Guerrero P, Muxika A, Zarandona I, de la Caba K (2019) Crosslinking of chitosan films processed by compression molding. Carbohydr Polym 206: 820-826.
- Xu L, Fu X, Su H, Sun H, Li R, et al. (2022) Corrosion and tribocorrosion protection of AZ31B Mg alloy by a hydrothermally treated PEO/chitosan composite coating. Prog Org Coatings 170: 107002.
- Ferrand A, Eap S, Richert L, Lemoine S, Kalaskar D, et al. (2014) Osteogenetic properties of electrospun nanofibrous PCL scaffolds equipped with chitosan‐based nanoreservoirs of growth factors. Macromol Biosci 14(1): 45-55.
- Eap S, Keller L, Schiavi J, Huck O, Jacomine L, et al. (2015) A living thick nanofibrous implant bifunctionalized with active growth factor and stem cells for bone regeneration. Int J Nanomedicine 10: 1061-1075.
- Chen YH, Tai HY, Fu E, Don TM (2019) Guided bone regeneration activity of different calcium phosphate/chitosan hybrid membranes. Int J Biol Macromol 126: 159-169.
- Murali VP, Fujiwara T, Gallop C, Wang Y, Wilson JA, et al. (2020) Modified electrospun chitosan membranes for controlled release of simvastatin. Int J Pharm 584: 119438.
- Tanaka CB, Lopes DP, Kikuchi LNT, Moreira MS, Catalani LH, et al. (2020) Development of novel dental restorative composites with dibasic calcium phosphate loaded chitosan fillers. Dent Mater 36(4): 551-559.
- Râpă M, Gaidau C, Mititelu TL, Berechet MD, Berbecaru AC, et al. (2021) Bioactive collagen hydrolysate-chitosan/essential oil electrospun nanofibers designed for medical wound dressings. Pharmaceutics 13(11): 1939.
- Nejaddehbashi F, Hashemitabar M, Bayati V, Abbaspour M, Moghimipour E, et al. (2019) Application of polycaprolactone, chitosan, and collagen composite as a nanofibrous mat loaded with silver sulfadiazine and growth factors for wound dressing. Artif Organs 43(4): 413-423.
- Baghaie S, Khorasani MT, Zarrabi A, Moshtaghian J (2017) Wound healing properties of PVA/starch/chitosan hydrogel membranes with nano Zinc oxide as antibacterial wound dressing material. J Biomater Sci Polym Ed 28(18): 2220-2241.
- Ahmed R, Tariq M, Ali I, Asghar R, Noorunnisa Khanam P, et al. (2018) Novel electrospun chitosan/polyvinyl alcohol/zinc oxide nanofibrous mats with antibacterial and antioxidant properties for diabetic wound healing. Int J Biol Macromol 120: 385-393.
- Ding X, Zhao L, Khan IM, Yue L, Zhang Y, et al. (2022) Emerging chitosan grafted essential oil components: A review on synthesis, characterization, and potential application. Carbohydr Polym 297: 120011.
- Yang X, Lan W, Sun X (2023) Antibacterial and antioxidant properties of phenolic acid grafted chitosan and its application in food preservation: A review. Food Chem 428: 136788.
- Ilic IK, Meurer M, Chaleawlert-Umpon S, Antonietti M, Liedel C (2019) Vanillin decorated chitosan as electrode material for sustainable energy storage. RSC Adv 9(8): 4591-4598.
- Chugh B, Singh AK, Poddar D, Thakur S, Pani B, et al. (2020) Relation of degree of substitution and metal protecting ability of cinnamaldehyde modified chitosan. Carbohydr Polym 234: 115945.
- El-Shorbagy HG, El-Kousy SM, Elwakeel KZ, El-Ghaffar MAA (2021) Eco-friendly chitosan condensation adduct resins for removal of toxic silver ions from aqueous medium. J Ind Eng Chem 100: 410-421.
- dos Santos Cardoso C, Vitali L (2021) Chitosan versus chitosan-vanillin modified: An evaluation of the competitive adsorption of five emerging contaminants. Water Air Soil Pollut 232(5): 179.
© 2023 Didem Demir. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






