- Submissions

Full Text
Polymer Science: Peer Review Journal
Porous Polymer Scaffolds for the Design of Functional Hybrid Materials and Sustainable Metal Catalysts
Antonio Buonerba and Alfonso Grassi*
Department of Chemistry and Biology, University of Salerno, Italy
*Corresponding author:Alfonso Grassi, Department of Chemistry and Biology, University of Salerno, Via Giovanni Paolo II-132, 84084, Fisciano (SA), Italy
Submission: May 01, 2023;Published: May 26, 2023

ISSN: 2770-6613 Volume5 Issue1
Abstract
This mini-review focuses the attention on the use of nanoporous organic polymers as support of inorganic nanoparticles for the design of metal catalysts active in organic synthesis. The porosity of the support enhances the performance of the metal catalyst, concentrating the reactants at the catalytic sites as a novel example of confined catalysis. Noteworthy metal nanoparticles embedded in nanoporous polymer support have been processed under the form of powder, membrane and monolith in which they found application in batch catalysis and flow chemistry, providing targeted examples of sustainable and green reaction pathways.
Keywords:Polymer; Nanomaterials; Metal catalysts; Nanoporous
Introduction
Hybrid nanomaterials are one of the most intriguing, stimulating, and exciting research fields in material science and heterogeneous catalysis [1]. These systems typically consist of an organic phase, a host polymer matrix or small guest molecules, compounded with an inorganic material to produce a functional structure defined at a nanometric scale. Two of the most representative examples are inorganic nanoparticles dispersed in an organic polymer phase and functional organic molecules trapped in porous scaffolds (e.g., MOF or zeolites) [2,3]. They are often characterized by extraordinary physical and chemical properties resulting from a synergistic effect of the two phases interacting throughout weak (Coulomb forces, London dispersion forces, hydrogen bonds, dipole-dipole forces, π-π stacking) or strong (Lewis acid-base, covalent, or ionic covalent bonds) chemical bonds; the final properties often differ significantly from those of a simple mixture of the two or more components. Innovative advanced hybrid nanomaterials have thus found a considerable variety of practical applications in Medicine [4,5], Optics [6], Electronics [7], Sensors [8], energy conversion and Storage [9], Mechanics [10], Membranes [11], and finally, in Catalysis [12].
In the latter contest, Metal Nanoparticles (MNPs) embedded/stabilized by polymer scaffolds are emerging as a powerful tool in heterogeneous catalysis to design reusable metal catalysts suitable for a wide range of chemical reactions of interest for industry or fundamental studies [13]. At first sight, organic polymers fare worse than classical inorganic supports such as carbon materials (amorphous carbon, graphene, carbon nanotubes) [14], Metal Oxides (Titania, Ceria, Nano-Silica) to stabilize MNPs vs their aggregation and leaching because of lower thermal stability and worse performances under harsh reaction conditions (elevated reaction temperature; use of strong oxidant or reducing agents; swelling in organic solvents).
However, they offer several practical advantages:
(i) The hydrophilic and hydrophobic properties of the support can be tuned by
appropriate functionalization of the surface, making the catalyst impregnable in solvents
or organic reagents with different polarity;
(ii) Functional groups containing donor atoms can be
introduced via common organic reactions to stabilize the MNPs;
(iii) Covalent functionalization of the support with Brønsted
acid and base functionalities allows modulation of the pH of the
reaction medium, making unnecessary the further addition of
acids or bases often required for the catalytic performances;
(iv) Processing of the polymer matrix via conventional
procedures allows for the production of the catalysts under the
form of films, beads, and foams adequately addressed to the
reactor design making easy also the application of these metal
catalysts in flow chemistry [12,15,16].
An interesting case is that of Porous Organic Polymers (POPs), that can be obtained via different synthetic approaches among which some representative examples are sketched in (Figure 1).
Figure 1:Synthetic pathways leading to the formation of porosity in polymer scaffolds.
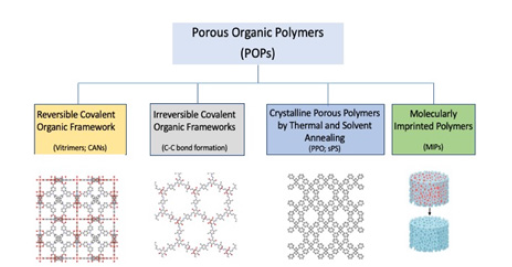
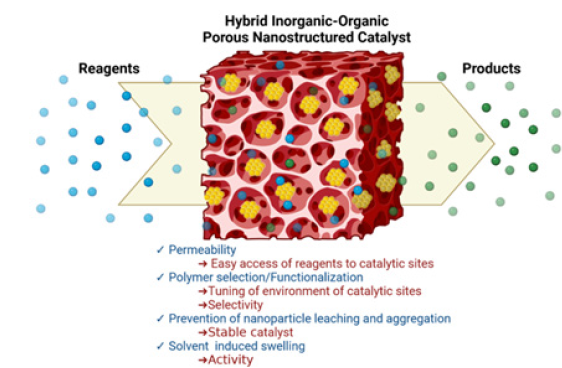
POPs make possible, when hosting catalytically active MNPs, multifunctional confined catalysis mimicking the active pocket in enzymatic catalysis (Figure 2) [13]. When large pores, such as macropores and mesopores, are produced, the enhanced specific surface area allows good permeability of the reagents penetrating the pores and having access to the metal catalyst site. In contrast, nanoporous polymer supports afford a larger specific surface area but lower accessibility to the pores, making possible size selectivity. Both these features allow the design of efficient catalytic processes.
Figure 2:Depiction of chemical transformation promoted by inorganic nanoparticles supported by porous organic polymers.
Survey Methodology
The authors used Scopus, Web of Science, and Google Scholar platforms to search for relevant documents published before April 2023. The keywords used were: “hybrid nanomaterials”, “porous polymer-supported metal catalysts” and “porous polymersupported gold nanoparticles”. The documents that provided significant advancements or insights on the application of porous polymer-supported metal catalysts were further considered and reviewed. The patents were excluded from the study.
Porous Polymer-Supported Inorganic Nanoparticles
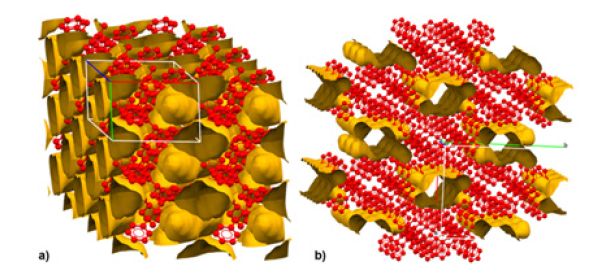
Gold Nanoparticles (AuNPs) currently stand out as very promising candidates in aerobic oxidation catalysis [17-19]. AuNPs incarcerated in crosslinked amorphous polystyrene matrix (AuNPs-PI) resulted very effective in alcohol oxidation to carbonyl derivatives in the presence of Brønsted bases under mild conditions (T=298K; PO2=1 bar) [20]. A zero-order kinetics for the alcohol to be oxidized, and an induction time of 15min were both observed and interpreted as the confirmation of diffusion-controlled kinetics resulting from the slow permeation of the alcohol through the amorphous phase of the polymer support [21]. Interestingly, AuNPs embedded in porous crystalline polystyrenic matrix (AuNPs-sPSB) with a specific area of 4-24m2/g and nanopore size of ≈2nm showed (Figure 3), under the same conditions, no induction time and firstorder kinetics in alcohol oxidation to prove the ready accessibility of the reagent to the metal catalyst determining higher catalytic activity [22].
Figure 3:Nanoporous crystalline forms of syndiotactic polystyrene: δ (a) and ε (b) forms, respectively, with isolated voids and nanochannels highlighted.
Noteworthy, high selectivity in alcohol oxidation was also assessed. Aliphatic alcohols, which do not penetrate the cavities and channels of the crystalline polymer phase, showed zero-order kinetics and lower oxidizability than the alcohols that this can do. Following these findings, aerobic oxidative esterification of Cinnamyl Alcohol (CA) [23] and 2,5-Hydroxymethyl Furfural (HMF) [24] to the corresponding alkyl esters was successfully obtained. Cascade reactions are one the main sustainable approach in organic synthesis where the reaction product of the first reaction feeds the subsequent reactions, carried out in the same batch reactor, without the need of isolation and purification steps of the products. The selective oxidation of the hydroxy functionality of CA and HMF in the presence of alkyl alcohols such as methanol, 1-butanol, 1-hexanol that are not oxidized, produced cinnamaldehyde or of 2,5-diformylfuran in the first reaction step; the nucleophilic attack of the alkyl alcohol to the carbonyl of the aldehydes, followed by a further oxidation of the resulting emiacetal intermediate produced alkyl cinnamates and dimethyl furandi carboxylate in high yields and excellent selectivity without observing the formation of cinnamic acid and 2,5-furandicarboxylic acid. Also in this case the kinetic order is e.g. zero for the alkyl alcohol and first-order for CA [24].
The above-mentioned gold catalyst AuNPs-sPSB porous was successfully applied in the intermolecular hydroamination reaction of anilines with phenylacetylenes in high yields and with excellent regio- and stereo-selectivity. The performances of the AuNPssPSB catalyst exceed those of the other commercial gold catalysts because of the nanoporous polystyrenic support which allows excellent activity, thermal stability and recyclability of the catalyst. The study of the reaction mechanism, carried out via kinetic investigation supported by DFT modelling, allowed determining interesting features of the hydroamination reaction pathway [25]. On the same topic AuNPs embedded in a highly porous poly(2,6- dimethyl-1,4-phenylene) (PPO) matrix, with a specific area of 4m2/g, better performed in benzyl alcohol oxidation. The reaction of benzyl alcohol with oxygen at atmospheric pressure afforded benzoic acid (selectivity>98%; TOF=2.45 molAlcohol/molAuh) or benzaldehyde (selectivity>99.9%; TOF=0.80 molAlcohol/molAuh) depending on the presence or absence of water in the reaction media [26]. Photocatalytic hydroxylation of benzene to phenol in presence of H2O2 oxidant has been studied using a photoreactive polymer composite based on N-doped TiO2 (N-TiO2) embedded into a monolithic syndiotactic polystyrene aerogel (N-TiO2/sPS, 10/90w/w). The polymer incarcerated N-TiO2 showed higher phenol selectivity (98%) and yield (57%) compared to bare N-TiO2 under visible light in acidic conditions (pH=2). The N-TiO2/sPS catalyst was recovered from the aqueous solution containing the reaction products and reused several times without significant loss of photo reactivity. This example represents a “proof of concept” of innovative green processes for the selective oxidation of aromatic hydrocarbons under mild conditions using a porous polymerembedded TiO2 catalyst [27].
AuNPs were produced in crosslinked Polymeric Inclusion Membranes (PIMs) and Polymeric Nanoporous Membranes (PNMs) composed of cellulose triacetate, 2-nitrophenoloctylether and Aldgen 364 or Alaniene 336 (tertiary amines); the average size of the AuNPs was of 37nm for PIMs and 2.9nm for PNMs. These gold catalysts allowed the reduction of 50M aqueous solution of 4-nitrophenol within 25min (PIMs) and 120min (PNMs) using NaBH4 as a reductant. These results rival those of the most efficient gold catalyst under batch conditions suggesting that the porous polymer support can produce an efficient catalyst under the form of a polymeric membrane [28]. Palladium Nanoparticles (PdNPs) of average size in the range of 6.6-13nm were successfully prepared using porous starch with a surface area of 177m2g-1 and average pore size of 8.2nm. A Pd content between 2.5 and 3 wt% was found to be the optimum for the balance of desirable textural properties and catalytic activity.
The starch-supported PdNPs catalysts were tested in the microwave-assisted Heck of iodobenzene with methylmethacrylate and styrene, Suzuki reaction of benzene boronic acid with bromobenzene, and Sonogashira reaction of bromobenzene with phenylacetylene. In all tested reactions, the starch-supported PdNPs performed better than the PdNPs supported on silica. The properties of the PdNPs are reflective of the unique porous environment that these novel polysaccharide-derived porous materials provide, allowing access to a wide range of surface chemistries, thus facilitating the preparation of metal nanoparticles of controllable size and nature. This palladium catalyst is also reusable, preserving the catalytic activity after four reuses [29].
Heterogeneous catalysis usually occurs at the surface of small MNPs. Despite the high surface area to volume ratio, only a few of the metal atoms are catalytically active, determining a reduced metal atom utilization, lower than that of homogeneous molecular catalysts where typically 100% of the metal atoms are active in the catalytic process, a figure that may be orders of magnitude higher than that of heterogeneous catalysts [30,31]. To overcome this drawback, one of the emerging research areas at the cutting edge in heterogeneous catalysis is the Single-Atom Catalysis (SAC), where the reusability of the metal catalyst is preserved along with the high metal atom utilization [10,11]. The increase of the metal atom utilization value of a catalyst is particularly important for heterogeneous catalysts based on platinum group metals (PGM) such as Pd, Pt, Rh, Ir or Ru. A spectacular example of SAC applied in sustainable catalysis is that of Ir embedded in a porous polyamine scaffold applied in CO2 reduction [32]. A porous organic polymer with aminopyridine functionalities (AP-POP), with mesopores having an average size of 7.6nm and a surface area of 43m2/g, was designed to fabricate a stable, atomically dispersed Ir catalyst. This Ir-based SAC exhibits excellent catalytic activity during the liquid phase hydrogenation of CO2 to formate, with a TON value as high as 25,135, representing the best performance for a heterogeneous conversion of CO2 to formate and well compare the best Ru and Ir homogenous catalysts in the field. The chemical structure of the Ir-SAC is analogous to that of a homogeneous mononuclear catalyst, representing an intermediary between heterogeneous and homogeneous catalysis [32].
Conclusion
The above reported selected examples aim to highlight and substantiate the peculiar properties of the inorganic nanoparticles embedded in porous polymer matrices. These hybrid nanomaterials have been prepared under the form of powder, fiber, membrane, monolith and applied in differ fields of sustainable catalysis showing high catalytic activity, selectivity and thermal stability. Moreover, they were applied in batch condition, or under the form of membrane and monolith in flow chemistry, with conventional heating or microwave irradiation, providing moderate to excellent performances. In our opinion, some of the above cited examples pave the way to novel research fields in catalysis that until a few decades ago seemed to be a peculiar prerogative of porous inorganic supports such as mesoporous silica, zeolites, MOF and others. The careful choice of the porous polymer support and of the inorganic counterpart, the tuning of their morphology and chemical physical properties, the identification of the optimal reaction conditions, all concur to valorize these hybrid nanomaterials in catalysis determining high activity and selectivity in organic transformation of relevance for industry, even disclosing novel reaction pathway, unconceivable with conventional catalytic systems.
Acknowledgement
The authors are grateful for the funding from the Ministero dell’Università e della Ricerca MUR (PRIN2017 grant 2017WR2LRS: project title: “Beyond fossil fuels: CO2-only monomers and polymers for a circular economy (CO2-only)”) and from the Università degli Studi di Salerno (FARB Buonerba ORSA224812).
References
- Sadykov VA (É), (2019) Advanced nanomaterials for catalysis and energy. Elsevier, Netherlands.
- Yang D, Gates BC (2019) Catalysis by metal organic frameworks: perspective and suggestions for future research. ACS Catal 9(3): 1779‑
- Wang H, Wang L, Xiao FS (2020) Metal@Zeolite hybrid materials for catalysis. ACS Cent Sci 6(10): 1685‑
- Park W, Shin H, Choi B, Rhim WK, Na K, et al. (2020) Advanced hybrid nanomaterials for biomedical applications. Prog Mater Sci 114: 100686.
- Buonerba A, Lapenta R, Donniacuo A, Licasale M, Vezzoli E, et al. (2020) NIR multiphoton ablation of cancer cells, fluorescence quenching and cellular uptake of dansyl-glutathione-coated gold nanoparticles. Sci Rep 10(1): 11380.
- Brett MW, Gordon CK, Hardy J, Davis NJLK (2022) The rise and future of discrete organic-inorganic hybrid nanomaterials. ACS Phys Chem Au 2(5): 364‑
- Inwati GK, Kumar P, Swart HC (2022) Multifunctional properties of hybrid semiconducting nanomaterials and their applications. Nanoscale Compound Semiconductors and their Optoelectronics Applications. In: Pawade VB, Dhoble SJ, Swart HC (É), Woodhead Publishing, UK, pp. 315‑350.
- Fernandes T, Daniel-da-Silva AL, Trindade T (2022) Metal-dendrimer hybrid nanomaterials for sensing applications. Coord Chem Rev 460: 214483.
- Saleh TA (2022) Nanomaterials and hybrid nanocomposites for CO2 capture and utilization: environmental and energy sustainability. RSC Adv 12(37): 23869‑
- Kumar V, Kumar P, Deka R, Abbas Z, Mobin SM (2022) Recent development of morphology-controlled hybrid nanomaterials for triboelectric nanogenerator: A review. Chem Rec 22(9): e202200067.
- Balakrishnan M, Yadav S, Singh N, Batra VS (2022) Membrane-based hybrid systems incorporating nanomaterials for wastewater treatment. Nano-Enabled Technologies for Water Remediation. In: Kaleekkal NJ, Mural PKS, Vigneswaran S (É), Elsevier, Netherlands, pp. 71‑144.
- Chongdar S, Bhattacharjee S, Bhanja P, Bhaumik A (2022) Porous organic–inorganic hybrid materials for catalysis, energy and environmental applications. Chem Commun 58(21): 3429‑
- Buonerba A, Grassi A (2021) Trends in sustainable synthesis of organics by gold nanoparticles embedded in polymer matrices. Catalysts 11(6): 714.
- Lam E, Luong JHT (2014) Carbon materials as catalyst supports and catalysts in the transformation of biomass to fuels and chemicals. ACS Catal 4(10): 3393‑
- Sun Q, Dai Z, Meng X, Xiao FS (2015) Porous polymer catalysts with hierarchical structures. Chem Soc Rev 44(17): 6018‑
- Zhang Y, Riduan SN (2012) Functional porous organic polymers for heterogeneous catalysis. Chem Soc Rev 41(6): 2083‑
- Hutchings GJ (2018) Heterogeneous gold catalysis. ACS Cent Sci 4(9): 1095‑
- Corma A, Garcia H (2008) Supported gold nanoparticles as catalysts for organic reactions. Chem Soc Rev 37(9): 2096‑2
- Stratakis M, Garcia H (2012) Catalysis by supported gold nanoparticles: beyond aerobic oxidative processes. Chem Rev 112(8): 4469‑
- Miyamura H, Matsubara R, Miyazaki Y, Kobayashi S (2007) Aerobic oxidation of alcohols at room temperature and atmospheric conditions catalyzed by reusable gold nanoclusters stabilized by the benzene rings of polystyrene derivatives. Angew Chem Int Ed Engl 46(22): 4151‑415
- Lucchesi C, Inasaki T, Miyamura H, Matsubara R, Kobayashi S (2008) Aerobic oxidation of alcohols under mild conditions catalyzed by novel polymer-incarcerated, carbon-stabilized gold nanoclusters. Adv Synth Catal 350(13): 1996‑
- Buonerba A, Cuomo C, Ortega Sánchez S, Canton P, Grassi A (2012) Gold nanoparticles incarcerated in nanoporous syndiotactic polystyrene matrices as new and efficient catalysts for alcohol oxidations. Chem Eur J 18(2): 709‑
- Buonerba A, Noschese A, Grassi A (2014) Highly efficient direct aerobic oxidative esterification of cinnamyl alcohol with alkyl alcohols catalyzed by gold nanoparticles incarcerated in a nanoporous polymer matrix: A tool for investigating the role of the polymer host. Chem Eur J 20(18): 5478‑
- Buonerba A, Impemba S, Litta AD, Capacchione C, Milione S, et al. (2018) Aerobic oxidation and oxidative esterification of 5-hydroxymethylfurfural by gold nanoparticles supported on nanoporous polymer host matrix. ChemSusChem 11(18): 3139‑
- Dentoni Litta A, Buonerba A, Casu A, Falqui A, Capacchione C, et al. (2021) Highly efficient hydroamination of phenylacetylenes with anilines catalyzed by gold nanoparticles embedded in nanoporous polymer matrix: Insight into the reaction mechanism by kinetic and DFT investigations. J Catal 400: 71‑
- Buonerba A, Noschese A, Capacchione C, Grassi A (2022) Gold nanoparticles supported on poly(2,6-dimethyl-1,4-phenylene oxide) as robust, selective and cost-effective catalyst for aerobic oxidation and direct esterification of alcohols. ChemCatChem 14(4): e20220033.
- Vaiano V, Sacco O, Sannino D, Ciambelli P, Longo S, et al. (2014) N-doped TiO2/s-PS aerogels for photocatalytic degradation of organic dyes in wastewater under visible light irradiation. J Chem Technol Biotechnol 89(8): 1175‑
- Mora-Tamez L, Esquivel-Peña V, Ocampo AL, Rodríguez de San Miguel E, Grande D, et al. (2017) Simultaneous AuIII extraction and in situ formation of polymeric membrane-supported Au nanoparticles: A sustainable process with application in catalysis. ChemSusChem 10(7): 1482‑
- Budarin VL, Clark JH, Luque R, Macquarrie DJ, White RJ (2008) Palladium nanoparticles on polysaccharide-derived mesoporous materials and their catalytic performance in C-C coupling reactions. Green Chem 10(4): 382‑
- Li J, Stephanopoulos MF, Xia Y (2020) Introduction: heterogeneous single-atom catalysis. Chem Rev 120(21): 11699‑
- Wang A, Li J, Zhang T (2018) Heterogeneous single-atom catalysis. Nat Rev Chem 2(6): 65‑
- Shao X, Yang X, Xu J, Liu S, Miao S, et al. (2019) Iridium single-atom catalyst performing a quasi-homogeneous hydrogenation transformation of CO2 to formate. Chem 5(3): 693‑
© 2023 Alfonso Grassi. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






