- Submissions

Full Text
Polymer Science: Peer Review Journal
Conditions of Production and Some Properties of Medical Films Based on Polyvinyl Alcohol
Elena Grehneva*, Elena Geraskina, Tatiana Kudryavtseva and Ekaterina Barteneva
Kursk State University, Research Laboratory of Organic Synthesis Kursk, Russia
*Corresponding author:Elena Grehneva, Kursk State University, Research Laboratory of Organic Synthesis Kursk, Russia
Submission: April 21, 2023;Published: May 01, 2023

ISSN: 2770-6613 Volume5 Issue1
Abstract
This paper examines the prospects of using polyvinyl alcohol as a unique binding filler, ideally suited for the creation of cutting-edge test materials and wound dressings. The conditions for obtaining polymeric medical films based on polyvinyl alcohol were revealed, as well as the investigation of certain physicochemical properties of the resulting films. The optimal polymer concentration in the solution used for film formation was determined. A composition that incorporates a cross-linking agent and a plasticizer was selected, providing the films with water resistance, elasticity, softness, and flexibility. Water absorption, swelling, and elasticity were assessed for a wide range of film compositions. The potential application of these developed films as long- lasting wound dressings was demonstrated.
Keywords:Polymer coatings; Polyvinyl alcohol; Cross-linking; Swelling; Water absorption
Abbreviations:PVA: Polyvinyl Alcohol; GA: Glutaraldehyde; PEG: Polyethylene Glycol
Introduction
Polymers have long been used in modern medical practice with considerable success. Their primary application lies in creating new forms of pharmaceuticals, such as microcapsules, hemostatic sponges, and medical films for various purposes. The simplicity and high technological nature of films allow their use not only as pharmaceutical agents but also as wound dressing materials [1,2]. In 1970, polymer films were officially introduced as a substitute for fast-dissolving tablets [3]. In the wound dressing market, an entire group of “medicinal films” has been identified, justifying the relevance of their use and the interest in improving this form of pharmaceutical release [4]. Ophthalmological and antiadhesion medicinal films are particularly popular [5], and their use as wound dressings is considered promising [6]. Such wound dressings are biodegradable, eliminating the need for additional surgeries, and in cases where an active substance is included in the polymer matrix, a controlled and gradual release of the pharmaceutical agent into the human body occurs [7,8]. As a result, the use of such coatings significantly simplifies postoperative wound care, contributing to the successful completion of surgical procedures. Currently, numerous polymer drug films have been developed, which possess various effects: Antimicrobial, Antiviral, Ammunomodulatory, etc. [9]. Heparin, dipyridamole, diclofenac, levofloxacin, and others are introduced as active pharmaceutical ingredients in such films [10,11].
Polymer indicator films with pH-indicative properties have also gained considerable recognition [12,13]. Films of this nature can be used for diagnosing pathological (and other) processes accompanied by changes in the pH of biological fluids. The possibility of using PolyVinyl Alcohol (PVA) as the sole polymer for creating medical coatings is due to its availability, low cost, and non-toxicity. Furthermore, PVA is permitted for use in the medical and food industries [14]. In the literature, there are sporadic studies dedicated to the application of PVA for the creation of medical coatings, both individually [15,16] and in combination with other polymers [17-19]. However, an in-depth study of the dependence of the properties of film coatings based on PVA on their composition has not been found in the literature sources. One of the objectives of this research is to create a universal set of matrices based on PVA with varying solubility to develop a wide range of therapeutic and diagnostic film coatings.
Materials and Methods
In this study, we used the following materials: PolyVinyl Alcohol (PVA) - Alfa Aesar CAS Number: 9002-89-5: low viscosity (Mw 10,000-26,000), medium viscosity (Mw 57,000-66,000), and high viscosity (Mw 88,000-97,000); GlutarAldehyde (GA)-CAS: 111-30- 8; Glyoxal (trimer dihydrate) crystalline–CAS: 4405-13-4; Glycerin- CAS: 56-81-5; PolyEthylene Glycol 400 (PEG-400)-CAS: 25322-68- 3.
Preparation of film coatings
A 3% aqueous solution of PVA was mixed with a crosslinking agent, either glutaraldehyde or glyoxal, and a plasticizer, either glycerin or PEG-400. The resulting solution was degassed in a vacuum desiccator DN 250 at a pressure of 15-20kPa for 20 minutes. The solution was poured into glass molds to achieve a layer thickness of 7mm. The material was dried in a drying oven, gradually increasing the temperature from 30 to 80 °C. Films with a thickness of 32 to 35 micrometers were obtained.
Methods for investigating the structure and properties of film coatings
Elasticity was determined according to GOST - 6806-73 using a set of cylindrical rods with diameters of 5, 6, 8, 10, 12, 15, 16, 20, and 25mm, as well as flat rods with rounded tops and a rounding diameter of 1, 2, 3, 4mm. The polymer film was placed on a larger diameter rod (25mm), tightly pressed, and smoothly bent around the object. After bending, the film was examined under a microscope for the presence of fractures and cracks. If no defects were found, the film was bent sequentially from larger diameter rods to smaller ones until defects were detected. To determine the swelling, samples of circular shape and equal size (Ø25mm) were filled with 20ml of distilled water. After specific intervals of time (from 10 to 300 seconds), the sample was removed from the water, excess moisture was removed, and the diameter of the film material was measured. Linear swelling was determined using the formula:
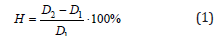
Where H is the swelling, %; D2 is the diameter of the swollen sample, mm; D1 is the diameter of the original sample, mm.
To determine water absorption, film samples weighing (0.1- 0.2g) were placed in a limited volume of distilled water (30ml). Every 30 seconds, the sample was removed from the water, excess moisture was removed, and it was weighed on ShinkoDenshi HT- 120CE analytical balances. Water absorption was determined using the formula:
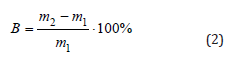
Where m1 is the mass of the initial polymer film, m2 is the mass of the polymer film after the test.
The residual content of glutaraldehyde in the film was determined by gas chromatography with flame-ionization detection and quantitative evaluation using the absolute calibration method on a “Crystal-2000M” chromatograph. The column used was a quartz, capillary HP-5 (30m/0.32mm/0.25μm), with a nitrogen flow rate (carrier gas) of 1.5ml/min, hydrogen flow rate of 20mL/ min, and air flow rate of 200mL/min. The evaporator temperature was 190 °C, the detector temperature was 220 °C, and the column temperature was 180 °C. The methanol extract, obtained by treating the film sample with ten times its volume of methanol, was subjected to the study.
Result and Discussion
Since PVA is widely used in creating various forms of pharmaceutical preparations, it seems quite promising to use this polymer to obtain film coatings based on it. PVA has excellent filmforming properties; however, its application is impossible without using plasticizers and cross-linking agents in its composition. Films obtained without the additional introduction of modifiers were brittle, inelastic, and quickly dissolved in water, which does not meet the requirements for medical films.
Film modification
Medical films, as pharmaceutical dosage forms, should primarily be elastic, strong, smooth, transparent, and slowly soluble in biological fluids [20]. To impart elasticity to the polymer film, a plasticizer is included, chosen from glycerin and PEG-400. Controlled solubility is provided by the introduction of a crosslinking agent (cross-linker) [21], such as glyoxal and glutaraldehyde [22-24]. During the conducted research, it was found that the inclusion of glycerin and PEG-400 in the polymer matrix increased the elasticity of the resulting polymer film. However, glycerin was more effective in imparting softness, flexibility, and elasticity. With an equal amount of plasticizer (10% of the polymer mass), the films containing glycerin exhibited greater elasticity. Elasticity assessment was carried out in accordance with GOST-6806-73. During the bending process, films containing PEG-400 developed microcracks and defects visible under a microscope. Films containing glycerin did not have such flaws. To choose the optimal amount of glycerin, a series of films was produced, each of which was controlled according to GOST 6806-73. The test results are presented in Table 1. At a glycerin content of 50% in the polymer matrix, flexible and elastic films were obtained that could withstand up to 50 bends around a rod with a diameter of 1mm without visually discernible defects.
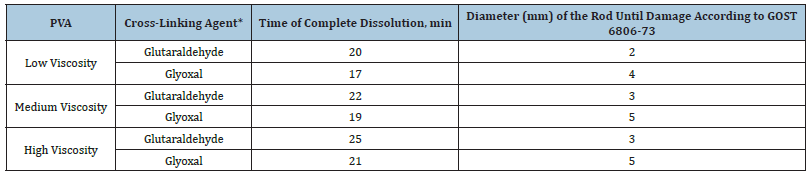
The most common cross-linkers for many polymers, including PVA, are glutaraldehyde and glyoxal. The study of cross-linked films showed that the introduction of glyoxal gives the films additional rigidity and does not provide the required cross-linking quality [25]. The test results are presented in Table 2.
Table 1:Results of elasticity determination.
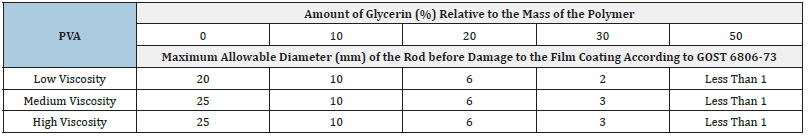
Table 2:Characteristics of films using different cross-linkers and containing 30% glycerin. *The cross-linking agent was added to the polymer matrix in an amount of 5% by weight of the polymer.
Water absorption and swelling
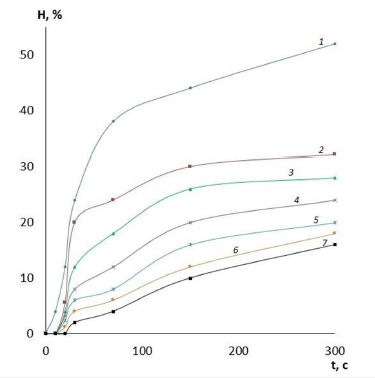
It is evident that by varying the amount of cross-linking agent, it is possible to change the main operational characteristics of the film coatings, including water absorption and swelling. These properties will determine the scope of application of these coatings for wound surfaces of different etiologies and, therefore, there is an opportunity to reduce the risk of various wound complications. The effect of the amount of cross-linking agent on the water absorption of films made of PVA with different viscosity is presented in Figures 1-3. It can be clearly seen that the quality of the polymer film depends directly on its composition. The higher the crosslinking agent content in the polymer matrix, the longer the dissolution time of the film and the less hygroscopic it is. This variation in composition makes it possible to produce coatings for wound surfaces with varying degrees of exudation. Another important characteristic of a wound coating is its degree of swelling or swellability. It determines the ability of the active substance contained in the film to migrate to the lesion site. The effect of the amount of crosslinker on film swelling is shown in Figures 4-6.
Figure 1:Water absorption of low viscosity PVA films with different GA contents: 1-2.5% GA; 2-5% GA; 3-10% GA; 4-15% GA; 5-20% GA; 6-25% GA; 7-30% GA.
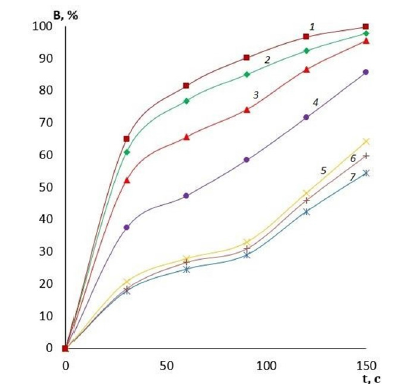
Figure 2:Water absorption of medium viscosity PVA films with different GA contents: 1-10% GA; 2-20% GA; 3-30% GA.
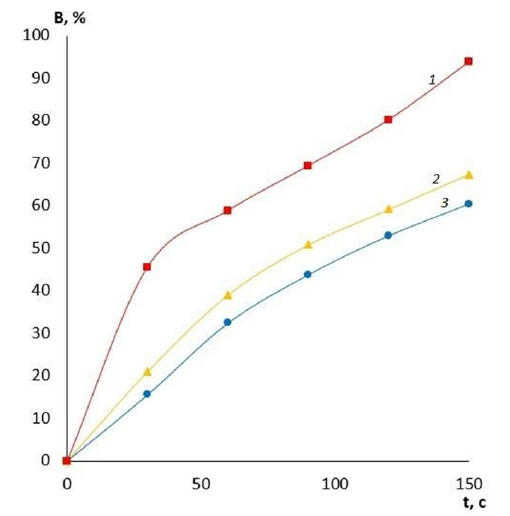
Figure 3:Water absorption of PVA high viscosity films with different GA contents: 1-10% GA; 2-20% GA; 3-30% GA.
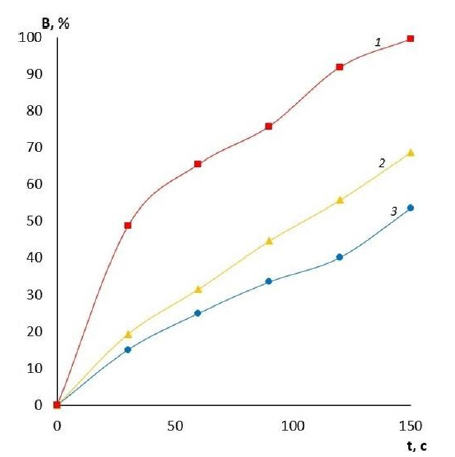
Figure 4:Dependencies of film swelling on time. Polymer: PVA low viscosity, GA content: 1-2.5%; 2-5%; 3-10%; 4-15%; 5-20%; 6-25%; 7-30%.
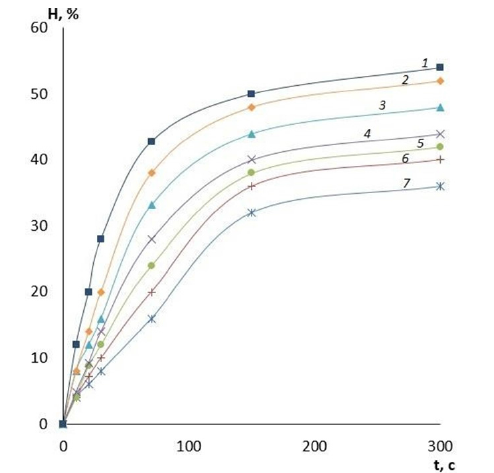
Figure 5:Dependence of film swelling on time. Polymer: PVA medium viscosity, GA content: 1-10% GA; 2-20% GA; 3-30% GA.
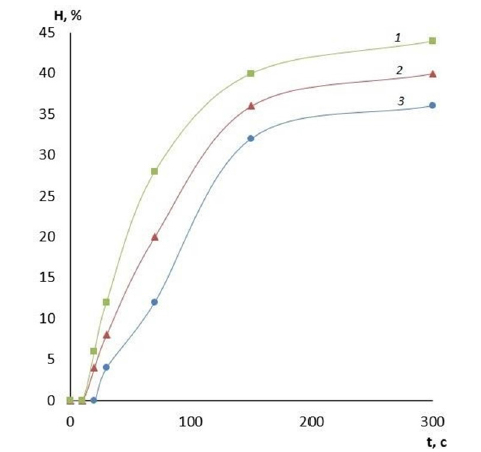
Figure 6:Dependence of film swelling on time. Polymer: PVA high viscosity, GA content: 1-10% GA; 2-20% GA;3- 30% GA.
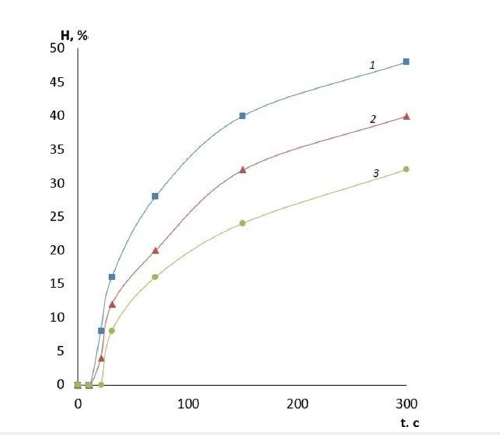
In summary, it appears promising to use polymeric matrices based on PVA as a depot for drug delivery. This is relevant both for postoperative wounds requiring hemostatic support and for other wounds contaminated with microorganisms where continuous antiseptic therapy is important [26]. Therefore, depending on the depth and severity of the wound process, it is possible to ensure the necessary rate of deposition of the active substance in the focus of the lesion. Swelling is precisely the indicator of the rate of prolonged release of the active substance, and the adjustable parameters here are the average molecular weight of the polymer used and the amount of cross-linking agent. The higher the content of glutaraldehyde in the polymer composition and the higher the viscosity of the initial polymer, the slower the swelling process of the polymer film and the more long-lasting the depot of the active substance will be. One of the main parameters for medical films is their safety. Therefore, all introduced cross- linking agent in the matrix should be consumed in the reaction or removed during drying. Gas chromatography method was used to evaluate the residual amount of GA in the film. It has been shown that when GA is added to the film in an amount exceeding 15% of the polymer weight, the amount of unreacted aldehyde can reach 5-7% of the initial amount. Therefore, to ensure the completeness of the reaction, subsequent use of GA as a cross-linker was carried out in an acidic environment. Hydrochloric acid was used in this study as a catalyst for the PVA cross-linking process. A composition based on high viscosity PVS with 5% GA addition was chosen for further investigations. The variable parameter was the content of hydrochloric acid. The obtained series of films was studied for swelling. The research results are presented in Figure 7. The presented results clearly demonstrate the role of acid in the crosslinking process. It can be seen that HCl acts not only as a catalyst for the cross-linking process with glutaraldehyde, but also as a reagent that independently reduces the solubility of PVA. This is evidenced by the gradual decrease in the swelling of the films with increasing concentration of HCl in the initial polymer solution. It is possible that the decrease in solubility in this case is realized due to intramolecular and intermolecular dehydration of PVA.
Figure 7:Swelling of polymer films with different ratios of acid and GA: 1-without acid; 2-1:0.5; 3-1:0.8; 4-1:1.0; 5-1:1.2; 6-1:1.5; 7-1:2.0.
Conclusion
The results of this study demonstrate the potential use of readily available and safe polymers such as polyvinyl alcohol as a matrix for the creation of medical coatings. The selected compositions of polymer compositions provide high elasticity, softness, smoothness, and transparency of the obtained films. By varying the amount of cross-linking agent, coatings with different degrees of water absorption and swelling were created, which determine the final properties and application range of these products in medicine. In the future, based on the developed formulations, it is planned to create and study drug films with active ingredients, as well as indicator films.
References
- Gonzalez JS, Maiolo AS, Hoppe CE, Alvarez VA (2012) Composite gels based on poly (vinyl alcohol) for biomedical uses. Procedia Materials Science 1: 483-490.
- Okada M (2002) Chemical syntheses of biodegradable polymers. Prog.Polym.Sci 27(1): 87-133.
- Kumar MS, Reddy M, Raju PN, Ravishankar K (2013) Formulation and evaluation of fast dissolving films of loratidine by solvent casting method. The Pharma Innovation J 2: 2.
- Vinnik YS (2015) Modern wound coatings in the treatment of purulent wounds -M. Surgery News 23: 5.
- Pankrusheva TA (2014) Polymeric drug films for the treatment of diseases of mucous membranes: Proceedings of the Orel State University. Series: "Natural sciences" 27(63): 212.
- Zheng C. Liu C, Chen H, Wang N, Liu X, et al. (2019) Effective wound dressing based on poly (vinyl alcohol) dextran-aldehyde composite hydrogel. International journal of biological macromolecules 132: 1098-1105.
- Chandra R, Rustgi R (1998) Biodegradable polymers. Prog. Polym. Sci 23: 1273-1335.
- Nair LS, Laurencin CT (2007) Biodegradable polymers as biomaterials. Prog. Polym. Sci 32(8-9): 762-798.
- Mayorova AV, Syisuev BB, Hanalieva IA, Vihrova IV (2018) Modern assortment, properties and prospects for improvement of dressings for the treatment of wounds K. Pharmacy and Pharmacology 6: 1.
- Fujimoto Т (1990) Chemotherapy.
- New HC (1989) Antimicrob Agents Chemotherapy.
- Huang TW, Lu HT, Ho YC, Lu KY, Wang P, et al. (2021) A smart and active film with tunable drug release and color change abilities for detection and inhibition of bacterial growth. Materials Science and Engineering: C 118: 111396.
- Oren U, Rääf CL, Mattsson S (2012) Gafchromic film as a fast visual indicator of radiation exposure of first responders. Radiat Prot Dosimetry 150(1): 119-123.
- Ogur EO, Jacobs D, Goodship V (2005) Polyvinyl Alcohol: Materials, Processing and Applications.
- Galya T, Sedlařík V, Kuřitka I, Novotný R, Sedlaříková J, et al. (2008) Antibacterial poly (vinyl alcohol) film containing silver nanoparticles: preparation and characterization. Journal of Applied Polymer Science 110(5): 3178-3185.
- Galya T, Sedlarik V, Kuritka I, Sedlarikova J, Saha P (2008) Characterization of antibacterial polymeric films based on poly (vinyl alcohol) and zinc nitrate for biomedical applications. International Journal of Polymer Analysis and Characterization 13(4): 241-253.
- Hajji S, Hamdi M, Jellouli K, Ayadi W, Nasri M, et al. (2017) Nanocomposite films based on chitosan–poly (vinyl alcohol) and silver nanoparticles with high antibacterial and antioxidant activities. Process Safety and Environmental Protection 111: 112-121.
- Feldman D (2020) Poly (vinyl alcohol) recent contributions to engineering and medicine. Journal of Composites Science 4(4): 175.
- Arakelov GG, Smirnova KS, Nichvolodin AG, Khizhnyak SD, Sokolov AV (2020) Composite films based on polyvinyl alcohol and Na-carboxymethylcellulose for separation purposes. SPB: Journal of Applied Chemistry 93: 1003-1008.
- Geraskina EA, Grekhneva EV, (2021) Some properties of medical films based on polyvinyl alcohol. E: Problems of theoretical and experimental chemistry: theses of the reports of the XXXI Russian Youth scientific conference with international participation dedicated to the 90th anniversary of the birth of Professor V. M. Zhukovsky 19.
- Gavaskar В, Kumar SV, Sharan G, Rao YM (2010) Overview n fast dissolving films. Int J of Pharmacy and Pharmaceutical Sciences 2(3): 29-33.
- Yakovlev AA, (2009) Cross-linkers and their use for the study of intermolecular interactions. Neurochemical Journal 3: 139-144.
- Bonewit-West K (2016) Clinical Procedures for Medical Assistant. (10th edn) p. 96.
- Garsuch V, Bretttreutz J (2010) Comparative investigations on different polymers for the preparation of fast-dissolving oral films. J of Pharmacy and Pharmacology 62(4): 539-545.
- Morandim‐Giannetti AA, Rubio SR, Nogueira RF, Ortega FS, Junior OM, et al. (2018) Characterization of PVA glutaraldehyde hydrogels obtained using central composite rotatable design (CCRD). Journal of Biomedical Materials Research 106(4): 1556-1566.
- Kim KJ, Lee SB, Han NV (1994) Kinetics of crosslinking reaction of PVA membrane with glutaraldehyde. Korean Journal of Chemical Engineering 11: 41-47.
© 2023 Elena Grehneva. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






