- Submissions

Full Text
Polymer Science: Peer Review Journal
Thermal Properties of HDPE/CaCO3 Materials
Zou Yiyi, Wang Kejian*, Wang Xuewei, Li Sihan and Low Zihao
College of Mechanical and Electrical Engineering, Beijing University of Chemical Technology, Beijing,100029, China
*Corresponding author:Wang Kejian, College of Mechanical and Electrical Engineering, Beijing University of Chemical Technology, Beijing,100029, China
Submission: March 14, 2023;Published: April 27, 2023

ISSN: 2770-6613 Volume4 Issue5
Abstract
The melting crystallization process of HDPE composite filled with CaCO3 powder was characterized by DSC. The results show that compared with HDPE and other HDPE composites with calcium carbonate ratio, when the content of CaCO3 is 30%, the crystallization entropy and melting entropy of HDPE are lower, the crystallinity is decreased, the melting point is slightly increased, the melting peak area is smaller, and the extrusion injection molding will be more energy saving.
Keywords:High density polyethylene; Inorganic particle packing; Thermal property
Introduction
Cheap inorganic materials are widely used as fillers in the plastics industry to improve the mechanical and thermal properties of polymers [1-3]. In particular, composites made from HDPE and calcium carbonate powder are widely used in films, large containers, logistics pallets, and drainage pipes. This paper studies the melting crystallization characteristics of HDPE/CaCO3 composites and reveals that adding calcium carbonate to HDPE composites can improve the thermal stability of materials, reduce the production cost of HDPE plastic products, and reduce the melting enthalpy during the production and processing of HDPE. This may mean that the addition of calcium carbonate is able to reduce the consumption of power resources during processing.
Experimental
Materials
High Density Polyethylene (HDPE), DMDA Type, 8007 (Shenhua Baotou Coal Chemical Co., LTD., China); A nearly spherical particle calcium carbonate powder with a maximum diameter of 0.1μm (Shandong Zhongtian Mining Co., LTD., China).
Composite material preparation
The CaCO3 and deionized water were mixed and stirred in the ratio of 1:1. Stand for 12h, filter out the water, and put the precipitated CaCO3 in the oven for drying at 100 ℃. High density polyethylene and dried calcium carbonate were evenly mixed in a high-speed mixer according to the mass ratio of 9:1, 8:2 and 7:3. The composite materials were added to the extruder to squeeze, and then the composite materials were cut and granulated. The finished granules were dried at 60 ℃ for 12h, and the samples were made for the determination of thermal properties.
Measurements
5-10mg samples were taken from the prepared composite materials and placed in a ceramic crucible. The crystallization behavior and melting characteristics of composites at 20 ℃ to 180 ℃ were studied by DSC-200F3, a German galloping scanning calorimeter, at the heating and cooling rates of 10K/min in nitrogen atmosphere.
Results and Discussion
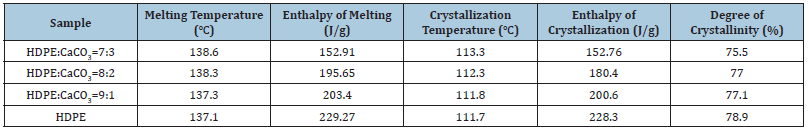
Curves (a) and (b) in (Figure 1) are DSC curves during the second temperature rise and temperature drop of the material. The curves show that HDPE and the HDPE material with CaCO3 added have only one melting peak and crystallization peak ,the melting point slightly increases with the increase of CaCO3 content, and the area of melting peak and crystallization peak decreases with the increase of CaCO3 content. The decrease of melting peak means that the consumption of power resources can be reduced in the injection molding or extrusion process. The crystallization peak shifts towards high temperature with the increase of CaCO3 content. The melting and crystallization behavior of materials is further analyzed, and the melting enthalpy, crystallization enthalpy and crystallinity in the second heating and cooling process are drawn in Table 1. With the increase of CaCO3 content, crystallization enthalpy and crystallinity will decrease, mainly because CaCO3 occupies the space of HDPE macromolecules during the cooling crystallization process, which restricts the movement of macromolecular chains [4]. The limitation degree of macromolecular conformation in the crystallization process can be described by crystallization entropy, according to the formula:
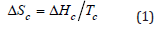
ΔHc is crystallization enthalpy and Tc is crystallization temperature.
Figure 1:DSC curves of the HDPE/CaCO3 composite at the second cooling and the second melting.
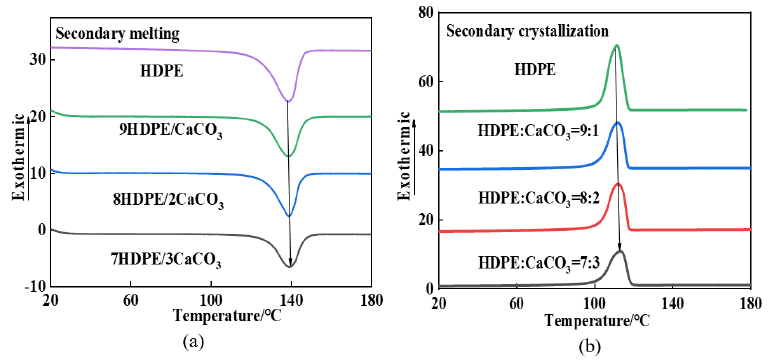
Table 1:Dsc characteristics of HDPE/CaCO3 composites.
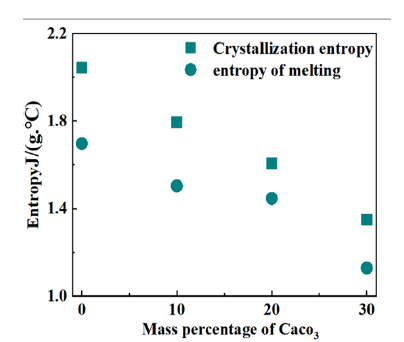
In the melting process, the enthalpy and entropy of melting decreased with the increase of CaCO3 content, indicating that the conformation of HDPE macromolecule is also limited during the melting process. It can be seen from (Figure 2) that crystallization entropy decreases with the increase of calcium carbonate content. Therefore, the greater the amount of calcium carbonate in the composite, the greater the reduction of macromolecular freedom in the crystallization process. The melting entropy decreases with the increase of calcium carbonate content. Therefore, the greater the content of calcium carbonate, the greater the reduction of macromolecular freedom in the smelting process, the higher the thermal stability of the composite.
Figure 2:Crystallization entropy and melting entropy of HDPE composites with different calcium carbonate contents.
Conclusion
a. When CaCO3 is added to HDPE, the HDPE material with 30% CaCO3 content can reduce the consumption of power resources. The heat insulation capacity of CaCO3 particles improves the heating capacity of the material, but the crystallinity of the material decreases. b. The melting peak area of HDPE composite can be reduced by DSC with more CaCO3 added. c. Fusion entropy and crystallization entropy can be used to quantitatively compare the conformation restriction degree of HDPE macromolecules in the melting and crystallization processes of composites with different contents of CaCO3.
References
- Rusu M, Sofian N, Rusu D (2001) Mechanical and thermal properties of zinc powder filled high density polyethylene composites. Polymer Testing 20(4): 409-417.
- Çoban O, Bora MÖ, Kutluk T (2018) Comparative study of volcanic particle and calcium carbonate filler materials in HDPE for thermal and mechanical properties. Polymer Composites 39(S3): E1900-E1907.
- Viljoen WD, Labuschagne FJWJ (2020) The thermal stability of highly filled high-density polyethylene quaternary composites: Interactive effects and improved measures. Polymer Testing 85: 106424.
- Elleithy RH, Ali I, Ali MA, Al-Zahrani SM (2010) High density polyethylene/micro calcium carbonate composites: A study of the morphological, thermal, and viscoelastic properties. Journal of Applied Polymer Science 117(4): 2413-2421.
© 2023 Wang Kejian. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






