- Submissions

Full Text
Polymer Science: Peer Review Journal
Substantiation of the Possibility of Obtaining Complex Including Nimesulide with γ-CD by Computer Modeling Methods
Barteneva Ekaterina1*, Grekhneva Elena1 and Efanov Kirill2
1Kursk State University, Kursk, Russia
2National Research Nuclear University MEPhI, Moscow, Russia
*Corresponding author:Barteneva Ekaterina, Kursk State University, Kursk, Russia
Submission: February 17, 2023;Published: March 20, 2023

ISSN: 2770-6613 Volume4 Issue5
Abstract
The problem of host-guest complexation based on cyclodextrin is a relevant area for research. These unique compounds have a number of features that allow improving a large number of biologically active substances known all over the world. Our work is a genuine experiment on the inclusion of a nimesulide molecule in the hydrophobic cavity of γ-cyclodextrin. The method of obtaining was selected individually, taking into account all factors affecting the process of complex formation. The obtained compound was analyzed using instrumental methods. In terms of theoretical approach, a powerful set of modern computational chemistry tools implemented in the Gaussian 16 software package was used. This method allows us to use not only semiempirical methods, but also the restricted Hartree-Fock method and the hybrid density functional-B3LYP method for calculations. The calculations were performed in the valence-split 6-31G basis set, which was complicated by adding polarization and diffusion functions as needed. The possible geometry of the γ-CD inclusion complex with nimesulide was predicted in various ways. In the field of supramolecular chemistry, there are not many works devoted to the synthesis of inclusion complexes with natural polymers-cyclodextrins.
Keywords:Inclusion complexes; Computer modeling; Geometry of active molecules; Nimesulide; Cyclodextrins
Abbreviations:BAS: Biologically Active Substance; CD: Cyclodextrin; γ-CD: Gamma-Cyclodextrin; RHF: Hartree-Fock Method; B3LYP: Density Functional Method
Introduction
Cyclodextrins are versatile natural high molecular weight compounds. The peculiarity of their structure lies in the internal hydrophobic cavity, which is able to contain various compounds, thereby forming an inclusion complex. The stability of these inclusion complexes is due to the formation of various noncovalent binding mechanisms of the “guest” molecule and the empty cavity of cyclodextrin. Such as hydrophobic or Van der Waals interactions. The value of this kind of compounds lies in the positive change in the properties of the “guest” molecule. Clathrate inclusion complexes are widely used in the pharmaceutical industry. Inclusion of biologically active substances of various classes in the CD cavity allows to increase their bioavailability and provides targeted action by their targeted delivery. It is this fact that determines the great interest of scientists in the study of complexation of the “host-guest” type. The choice of nimesulide as a “guest” molecule is explained from the perspective of the most common and available nonsteroidal anti-inflammatory agent used in modern medicine to treat people with rheumatologic diseases. The formation of a complex including nimesulide with γ-CD will increase the biological activity of this drug, reduce its therapeutic dose, and reduce the toxic effect on the patient’s body as a whole [1]. Thus, this study covers a large section of polymer chemistry in relation to the acute problem of increasing the bioavailability of various drugs.
Materials and Methods
Chemically pure reagents Molcan (CAS 51803-78-2) and AcrosOrganics (CAS 68168-23-0) were used to synthesize the inclusion complex of nimesulide with γ-CD. The complexes were obtained in two stages. At the first stage, the CD was dissolved in a minimum volume of water. Nimesulide solution in dichloromethane was then added under constant stirring to incorporate into the hydrophobic cavity of the CD macrocycle. Second step: their mostatting at 60 °C for 24 hours, followed by cooling to 5-10 °C. The cavitate precipitate formed as a result of the reaction was separated by filtration and dried in the air. The current analysis was performed by thin-layer chromatography. «Sorbfil» chromatographic plates, eluent-toluene: acetone: ethanol-10:3:2; eluent-ethanol: butanol: chloroform: water-5:4:4:1. Quantitative analysis of the obtained compounds was carried out by the method of calibration curve on a Shimadzu UV-1800 spectrophotometer. Quantitative analysis was also carried out by HPLC with a UV detector using a Waters MSD SQD - ESI chromatograph (reversephase HPLC; detector: spectrophotometric, 220nm; Acquity BEH C18 2.1mm×50mm*1.7um column; mobile phase: water (0.1 % formic acid) - acetonitrile (0.1 % formic acid); elution mode: gradient: 0.4ml/min). The obtained products were also evaluated by infrared spectroscopy. The IR spectra of the samples were taken on a Fourier spectrometer (“FMS 1201”, Russia). The interpretation and analysis of the obtained spectra was carried out using “OMNIC” software [2,3]. Molecular modelling of the studied compounds was carried out using the Gaussian16 software package. This software tool allows the full functionality of modern computer chemistry to be used to describe the characteristics of the proposed inclusion complex. The whole process proceeded by complicating the parameters in order to obtain the most objective result.
Results and Discussion
The steric factor largely determines the process of complex formation. Nimesulide has certain functional groups in its structure that may prevent the free entry or fixation in the internal cavity of the γ-CD. In addition, the structure of the γ-CD molecule known from the reference literature is idealized and does not reflect the reality [4]. In fact, the γ-CD molecule is not a perfect torus, as it undergoes various kinds of deformations under the influence of external factors and intramolecular rearrangements. The diameter of the nimesulide molecule was theoretically calculated as the length of the vector most distant from each other atoms and is 5.2 Å. This means that the diameter of the nimesulide molecule is commensurate with the ideal internal cavity size of γ-CD, known from the literature as 9.5 Å. Thus, based on the size correspondence of the active molecules, it can be assumed that the production of an inclusion complex is theoretically possible. However, the reasons outlined above can lead to a significant reduction in the diameter of the internal cavity of the γ-CD, up to and including its collapse [5].
Therefore, computer modelling techniques were applied to obtain more reliable structures of the initial reagents and to assess the feasibility of the reaction under study. The results obtained by optimizing the geometry of the reagents using the limited Hartree- Fock method led to the conclusion that steric hindrances exist in the reaction. However, the use of hybrid density functional methods in the calculation of molecular systems made it possible to verify the existence of the desired clathrate complex from a thermodynamic point of view [6]. In the next phase of the study, a conformational analysis of the γ-cyclodextrin-nimesulide molecular system was carried out using the Gaussian 16 software package tools. The calculation was carried out using the semi-empirical PM3 method, which allows a qualitative assessment of the main characteristics of the complex under study without much time. The result of the analysis is a curve of the total potential energy of the system as a function of the distance between the centers of mass of the molecules (Figure 1). In the first approximation, the resulting curve can be characterized as a visualization of the descent along the coordinate of the reaction of the inclusion complex under study. The search for local minima on the multidimensional potential energy surface of the system at each of its points will lead to the determination of the path of the process under study. In this case, the minimum on the curve (Figure 1) corresponds to the reaction product, the maximum to the transition state, and the points lying on the plateau on the right correspond to the reagents. It is not possible to judge the external factors necessary for the process from the results obtained, due to the deviation from the real reaction pathway. However, the presence of a deep minimum in the product area may indicate both the possibility of spontaneous complex formation under normal conditions and its stability.
Figure 1:Curve of the potential energy of a system as a function of the distance between the centers of mass of the molecules.
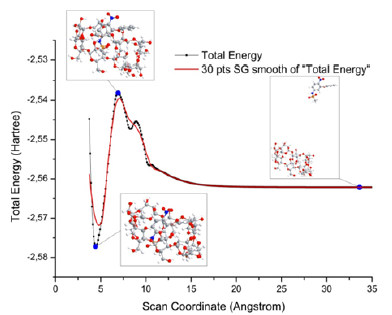
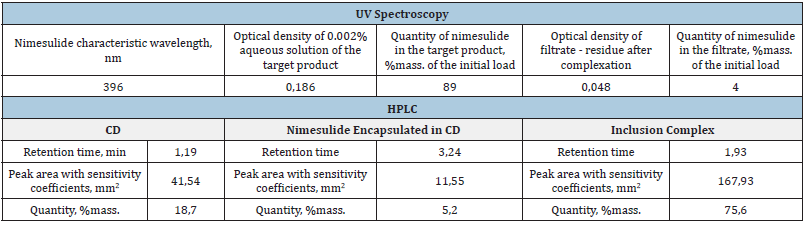
Optimal conditions for the complexation process were selected by varying various parameters such as mixing and settling time, temperature in the reaction mixture, solvent volumes. Quantitative assay methods showed incomplete binding of nimesulide to the clathrate complex. Part of the drug remains in the free form and precipitates surrounded by CD particles. However, the formation of the new compound was unequivocally confirmed by chromatographic methods as well as by infrared spectroscopy. Consequently, the technique described in this work allows us to obtain a mixture of the nimesulide clathrate complex and its micro capsulated in γ-CD form. Despite the fact that the size of the inner cavity of the γ-CD torus is commensurate with the diameter of the nimesulide molecule, the geometry of the molecules creates steric barriers and does not allow full completion of the complexation process. The IR spectra of the complex show a shift of some absorption bands related to nimesulide in the structure of the complex compared to the IR spectra of pure nimesulide. These are the normal vibrations of the benzene ring (1593cm-1) and the sulfur-containing functional group R-SO2-R (1284cm-1, 1154cm- 1). This fact indirectly confirms the participation of the indicated groups of atoms in the formation of intermolecular bonds with the atoms of the internal cavity of the CD. The same result is observed when creating and optimizing the structure of the clathrate complex by computer modeling methods. The amount of nimesulide in the isolated product was estimated by the method of calibration graph using UV/visible spectrophotometer spectral range. The optical density of the 0.02% solution of the product precipitate and the optical density of the filtrate at the wavelength characteristic of nimesulide were determined. However, this method detected both nimesulide bound to the complex and nimesulide encapsulated in the CD shell in the obtained product. It was possible to detect these forms of nimesulide separately by absolute normalization HPLC. Under elution conditions, CD and nimesulide forming microcapsules are detected separately. This makes it possible to separate the clathrate complex of nimesulide and its microencapsulated form. The results are given in Table 1. Consequently, the technique described in this work makes it possible to obtain a mixture of the nimesulide clathrate complex and its microencapsulated form in the γ-CD. Although the size of the internal cavity of the γ-CD torus is commensurate with the diameter of the nimesulide molecule, the geometry of the molecules creates steric obstacles and does not allow complete completion of the complexation process.
Table 1:Clathrate complex of nimesulide and its microencapsulated form.
Conclusion
This paper makes it clear that the steric factor and its consequences are of great importance in the host-guest complexation process. For each individual biologically active substance, a calculation must be made in advance by means of computer chemistry and then the process conditions must be selected. The synthesis of the inclusion complex of nimesulide with γ-CD can be extended to other drugs with anti-inflammatory functions. The existence of the inclusion complex has been proved by physico-chemical methods of analysis. Computer modelling plays a leading role in the study of the complexation process and helps us to see a clear picture of the interaction of active molecules.
References
- Gieroba B, Kalisz G, Sroka-Bartnicka A, Płazinska A, Płazinski W (2021) Molecular structure of cefuroxime axetyl complexes with α-, β-, γ- and 2-hydroxypropyl-β-cyclodextrins: Molecular modeling and Raman spectroscopy and imaging studies. Int J Mol Sci 22(10): 5238.
- Miranda GM, Santos VOR, Bessa JR, Teles YCF, Yahouédéhou SCMA (2021) Inclusive complexes of nonsteroidal anti-inflammatory drugs with cyclodextrins: A systematic review. Biomolecules 11(3): 361.
- Barteneva ES, Grekhneva EV (2022) Preparation and theoretical study of nimesulide inclusion complexes with β- and γ-cyclodextrins. Electronic Scientific Journal Auditorium of Kursk State University, Russia, 4(36): 1-4.
- Singh R (2010) Characterization of cyclodextrin inclusion complexes-A review. Journal of Pharmaceutical Science and Technology 2(3): 171-172.
- Grekhneva EV, Barteneva ES, Efanov KS (2022) Features of obtaining and modeling the structure of nimesulide clathrate complexes with β- and γ-cyclodextrins. Chem Proc pp. 1-8.
- Sahra K, Dinar K, Seridi A, Kadr M (2022) Investigation of inclusion of diclofenac with β- cyclodextrin: a molecular modeling approach. Struct Chem 26: 61-69.
© 2023 Barteneva Ekaterina. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






