- Submissions

Full Text
Polymer Science: Peer Review Journal
Influence of Matrix Type and Oil Phase Composition on the Stability of Polysaccharide- Based Nanoemulsions
Flavia Oliveira Monteiro Da Silva Abreu1,2*, Joice Farias Do Nascimento1, Taysse Holanda2, Rachel Menezes Castelo1, Roberta Bussons Rodrigues Valério3 and Maria Madalena de Camargo Forte2
1State University of Ceará, Center for Sciences and Technology/LAPONAT-Natural Sciences Program, Brazil
2Federal University of Rio Grande do Sul, Engineering School/LAPOL-PPGEM, Brazil
3Federal University of Ceará, Department of Analytical Chemistry and Physical Chemistry, Brazil
*Corresponding author:Flavia Oliveira Monteiro Da Silva Abreu, State University of Ceará, Center for Sciences and Technology/LAPONAT-Natural Sciences Program, Brazil. Federal University of Rio Grande do Sul, Engineering School/LAPOL-PPGEM, Brazil
Submission: November 21, 2022;Published: December 12, 2022

ISSN: 2770-6613 Volume4 Issue4
Abstract
Polymeric nanoemulsions are systems capable of protecting bioactive compounds, improving bioavailability and controlled release. Stable formulations must present uniform micellar dispersion with nanometric particle size and homogeneous morphology. Thus, a detailed analysis regarding oily and aqueous phase in nanoemulsions and their compositions is fundamental to meet the physicochemical stability criteria. For this study, we tested the matrices of alginate and chitosan as wall material and commercial soy oil and mineral oil as co-adjuvants along with the surfactant for the micelle’s composition. Nanoemulsions coated with alginate and with soy oil presented the best stability factors, with particle size around 200nm, morphological homogeneity, and high values of Zeta potential, being promising for controlled release.
Keywords:Nanoemulsion; Stability; Formulation
Introduction
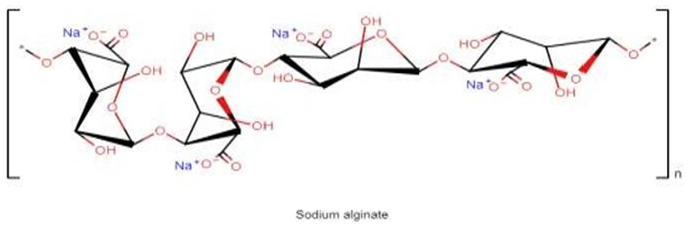
Nanoemulsions (NEs) are useful alternatives in the encapsulation of active principles, enabling the improvement of physical-chemical stability, the modulation of release rates, and bioavailability of such active principles. They are dispersions formed by two immiscible phases linked by a surfactant agent. Translucid emulsions have a micelle size of less than 200nm [1]. These systems emerge as a promising alternative for the delivery of various types of molecules [2]. Chalcones are aromatic ketones derived from flavonoids, which are highly electrophilic compounds. They are of natural origin and widely distributed in plants and with several biological activities proven and used in traditional medicine [3]. One possibility to obtain compounds with activity against microorganisms is the guided synthesis of new compounds that have some similarity with the compounds from natural products, thus avoiding the in vivo shortage of the molecule [4]. These compounds have low solubility, which hinders their absorption. This problem can be solved with the use of texture modifiers. The most targeted parameters for choosing a matrix for nanoemulsion production are the functionality that the encapsulation should provide to the product, release method, stability, and cost constraints. Therefore, polysaccharides are promising materials in the development of oil/water (O/W) nanoemulsions. They are being used to improve stability of nanoemulsions, because of their emulsifying and thickening capacity and as weighting agents, which also coat the individual droplets, reducing droplet movement preventing destabilization of nanoemulsions [5]. Among the polysaccharides, Alginate (Figure 1), which is a natural anionic polysaccharide extracted from brown algae, has been widely evaluated as a wall matrix for encapsulation of lipophilic compounds [6,7].
Figure 1:Chemical structure of sodium alginate.
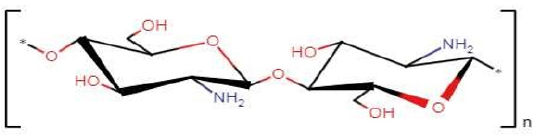
Chitosan (Cs) is a cationic biopolymer in acid conditions (Figure 2) and is formed from D-glucosamine and N-acetyl-Dglucosamine bound by β-glycosidic bonds. Several studies point out the potential as a matrix in nanoparticle systems [8], nanogels [9], and conventional emulsions [10]. Therefore, the present work seeks to evaluate the stability of polymeric nanoemulsions carrying chalcones, comparing different polymeric matrices and oil phase composition, to point out the most stable formulation for drug encapsulation and controlled release.
Figure 2:Chemical structure of chitosan.
Method
Materials
Sodium alginate (DINAMICA), chitosan (ÊXODO CIENTIFICA) surfactant Tween 80® (VETEC), commercial soy oil, mineral oil (NATULAB), synthetic chalcone 1,5-diphenylpenta-1,4-dien- 3-one (Acetone) was given by the Chemistry of Natural Products Laboratory of the State University of Ceará.
Preparation of the Nanoemulsions
Four oil-in-water nanoemulsions (O/W) were prepared by the high-speed homogenization method, adapted from Ribeiro et al. [11], by varying the type of oil and the matrix type in the nanoemulsion. First, the surfactant agent (Tween 80) was added to the chalcone at a mass ratio of 1:2 and the mineral or soy oil were added. Then, the oily phase was subjected to an ultrasonic vortex (model NI1059 - Vortex) at 1000rpm for 5min, forming the oil phase. The oil phase was slowly added with the aid of a syringe into the 100ml of the Cs solution (1% v/v in acetic acid) or alg (1% v/v in water) to form the nanoemulsions with a volume ratio with the aid of a mechanical homogenizer Model ULTRA380 of the Ultrastirrer brand at 18.000rpm over 3min. Table 1 indicates the composition and proportion of the materials used in the nanoemulsions production.
Table 1:Experimental conditions of nanoemulsions production with chalcone.

Characterizations
Visual analysis of the samples performed the stability of the nanoemulsions for 60 days. Where the appearance or not of creaming or sedimentation was verified, and if these changes occur, their volume is measured with the aid of a ruler. The optical microscopy test was performed in an Olympus CX31 optic microscope, where a drop of each formulation was placed on a slide and covered by a coverslip. The samples were viewed at 200x aim. Particle size, polydispersity index, and zeta potential characterizations were performed on a Malvern Zetasizer/ Nanoseries Z590. The nanoemulsion is diluted at a ratio of 1:100 and stirred continuously for 24 hours.
Results and Discussion
The instability of the nanoemulsion is undesirable and creates several problems in longer storage [12]. The samples didn’t demonstrate sedimentation, a clear sign of stability. Table 2 shows the creaming results for the samples analyzed after 60 days. Samples N2 and N1 presented lower creaming with values below 2.5%, therefore more stable than samples N3 and N4. The samples with lower creaming volume were prepared with a matrix of sodium alginate and vegetable oil. Overall, all showed a creaming percentage of less than 3.2%, so they all meet the stability criterion. Table 2 also shows particle size, Zeta Potential and Polydispersity Index values for the nanoemulsions. Particle size varied from 100 to 370nm, demonstrating an expressive difference in the size of the micelles by varying the matrix type. Alginate as a matrix produced nanoemulsions of desirable size below 200nm. McClements [13] reported that increasing particle size could increase the speed of creaming formation. In our study, in fact, samples N3 and N4, with chitosan as a matrix, presented higher particle size and also higher creaming index. The samples N1 and N2 presented the lowest particle sizes and achieved the lowest creaming formations. High values of Zeta Potential in modulus confer greater stability in nanoparticles once these values handle the charge on the surface of the particle. These values determine the behavior of particles in a liquid and the tendency in aggregation and/or flocculation. All formulations have values higher than +30mV or lower than -30mV, which gives them stability, indicating repulsion of adjacent particles [14]. The negative charges of the alginate emulsions are because of the deprotonation of the carboxyl group, while the positive charge of the samples produced with chitosan is because of the positive charges of the free amine groups.
Table 2:Particle size, zeta potential and polydispersity index results for nanoemulsion samples
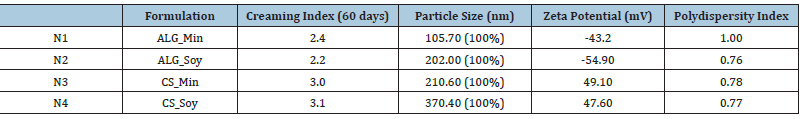
As mentioned, the most stable sample considering the Zeta Potential is the N2 prepared with sodium alginate and vegetable oil, because it has the highest value in modulus, followed by N3, N4 and N1. Figure 2 shows the morphological analysis of the nanoemulsions where it is possible to observe the dispersion of the droplets around the aqueous phase and some phenomena as in N1 the flocculation of particles which will confer a heterogeneity in the colloidal dispersion, which can be justified with its PdI value equal to 1.00. Sample N2 presented homogeneous size, conferring greater stability and smaller particles, which can be explained by its PdI value close to 0.7. Chitosan samples N3 and N4 present larger and heterogeneous sizes compared to the two samples already mentioned and a smaller number of micelles, which can be justified by the coalescence phenomenon.
Conclusion
Figure 3:Optical microscopy analysis of the samples: (a) ALG_Mineral oil – N1; (b) ALG_Soy oil - N2; (c) CS_Mineral oil – N3; (d) CS_soy oil- N4.

Nanoemulsions were prepared and the effect of polysaccharide and type of oil was investigated. The type of matrix and the type of oil influenced the particle size, where Chitosan and Soy oil increased the droplet size (Figure 3). Chitosan emulsions may be subjected to coalescence phenomenon. All samples showed stability in the creaming and Zeta Potential, although sample N2, having sodium alginate as polymeric matrix and vegetable oil in its oily phase obtained the best balance of properties, with lower creaming, desirable particle size and morphological homogeneity, being promising for nanoencapsulation and controlled release.
References
- Nascimento JF, Costa EF, Abreu FOM (2020) Characterizations of sodium alginate nanoemulsions with eucalyptus citriodora essential oil. Revista Coleta Científica 4(8): 15-22.
- Abhijit AD, Nagarsenker MS (2008) Parenteral micro-emulsions: An overview. Int J Pharm 355(1-2): 19-30.
- Rozmer Z, Perjesi P (2016) Naturally occurring chalcones and their biological activities. Phytochemistry reviews 15(1): 87-120.
- Umesha B, Basavaraju YB (2014) Synthesis and characterization of novel benzo [d] [1, 3] dioxole gathered pyrazole derivatives and their antimicrobial evaluation. Medicinal Chemistry Research 23(8): 3744-3751.
- Maphosa Y, Jideani VA (2018) Science and technology behind nano emulsions.
- Odriozola SI, Oms OG, Martín BO (2014) Nano emulsion-based delivery systems to improve functionality of lipophilic components. Frontiers in Nutrition 1(24).
- Sachan NK, Pushkar S, Jha A, Bhattcharya A (2009) Sodium alginate: the wonder polymer for controlled drug delivery. Journal of Pharmacy Research 2(7): 1191-1199.
- Casettari L, Illum L (2014) Chitosan in nasal delivery systems for therapeutic drugs. J Control Release 28(190): 189-200.
- Abreu FOMS, Oliveira EF, Paula HCB, Paula RCM (2012) Chitosan/cashew gum nanogels for essential oil encapsulation. Carbohydrate Polymers 89(4): 1277-1282.
- Xiong W, Ren C, Tian M, Yang X, Li J, et al. (2018) Emulsion stability and dilatational viscoelasticity of ovalbumin/chitosan complexes at the oil-in-water interface. Food Chemistry 252: 181-188.
- Ribeiro JC, Ribeiro WLC, Vasconcelos ALFC, Macedo ITF, Santos JML, et al. (2014) Efficacy of free and nanoencapsulated eucalyptus citriodora essential oils on sheep gastrointestinal nematodes and toxicity for mice. Veterinary Parasitology 204(3-4): 243-248.
- Sharma N, Bansal M, Visht S, Sharma PK, Kulkarni GT (2010) Nanoemulsion: A new concept of delivery system. Chronicles of Young Scientists 1(2): 2-6.
- McClements DJ (2004) Food emulsions: Principles, practices, and techniques. CRC Press, US.
- Larsson M, Hill A, John D (2012) Suspension stability: why particle size, zeta potential and rheology are important. Annual Transactions of the Nordic Rheology Society 20: p. 6.
© 2022 Roberta Bussons Rodrigues Valério. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






