- Submissions

Full Text
Polymer Science: Peer Review Journal
Polymeric Membranes for Micronanoplastic Sampling and Removal from Water Effluents
Junkal Landaburu-Aguirre* and Serena Molina
IMDEA Water Institute, Avenida Punto Com, 2, Alcalá de Henares, 28805 Madrid, Spain
*Corresponding author:Junkal Landaburu-Aguirre, IMDEA Water Institute, Avenida Punto Com, 2, Alcalá de Henares, 28805 Madrid, Spain
Submission: November 17, 2022;Published: December 06, 2022

ISSN: 2770-6613 Volume4 Issue4
Abstract
Micro and Nano Plastics (MNPs) are pollutants of increasing concern that are found in water samples such as marine environment and freshwater. Wastewater Treatment Plants (WWTPs) have been identified as one of the main dominant sources of Microplastics (MPs) in freshwater. Primary and secondary wastewater treatments remove the majority of MPs, achieving removal efficiencies of 98-99%. However, these studies have mainly monitored relatively big size MPs leaving the small size MNPs aside. Consequently, the release of MNPs from WWTP to the aquatic environment might be much more problematic than known to this day. The implementation of advanced treatment processes such as membrane technology can contribute to overcoming these limitations, by implementing them not only as a tertiary treatment, but also as a sampling method. This minireview shows the latest works related to the separation and removal efficiency of MNPs using pressure driven microfiltration and ultrafiltration membranes. In addition, membrane fouling mechanism by MNPs is explained showing that the hydrophobic interaction and the relation between particle size and membrane pore size have the main influence on it.
Keywords: Polymeric membranes; Micronanoplastics; Wastewater treatment; Fouling
Abbreviations:MNPs: Micro-Nano Plastics; WWTPs: Wastewater Treatment Plants; FTIR: Fourier Transform Infrared; MF: Microfiltration; UF: Ultrafiltration
Introduction
The plastic industry contributes to economic growth, having produced an output of 377 million Metric Tonnes (Mt) by 2020 [1]. This massive production has a great environmental impact. Around 78% of the plastics produced have been discarded either in landfills or elsewhere in the environment. Once in the environment, plastics undergo abiotic and biotic weathering processes that cause their degradation and fragmentation into smaller particles, commonly termed Microplastics (MPs; defined as fragments<5mm) and Nanoplastics (NPs, defined as fragments<1μm) [2,3]. The Micro-Nano plastics (MNPs) that are formed due to the fragmentation of larger plastics by physical, mechanical or biodegradation are called secondary MNPs. In addition, there are also primary MNPs, which are specifically manufactured in micro(nano)-size for use in personal care, cosmetics or synthetic textiles, etc. [4]. MP and NP particles are emerging pollutants of increasing concern. The ocean is estimated to already contain over 150Mt of plastics [5]. In addition, recent studies revealed that the abundance of MPs in freshwaters is comparable to that of marine environment [6]. Under this context, Wastewater Treatment Plants (WWTPs) have been identified as one of the main dominant sources of MPs in freshwater [7]. The MPs found in wastewaters consist for example of microbeads added in peeling lotions and toothpastes and synthetic fibers from textile and clothing [4]. In general terms, studies have indicated that wastewater treatments plants remove the majority of MPs, achieving removal efficiencies of 98-99% [8,9]. Among the conventional physico-chemical treatment methods used in the removal of these pollutants there are sand filtration [10], coagulation-flocculation [11], membrane bioreactor [12], electrocoagulation [13] and sorption [14]. Despite the high removal ability of the wastewater treatment technologies studied, the research efforts have been mainly limited to the quantification of relatively big size MPs, leaving small size MP and NPs out of the studied size spectrum. The evaluation of the removal capacity of Micronanoplastics (MNPs) by advanced water treatment technologies has been limited due to the lack of standardized methods for sampling, identification and quantification of MNPs from wastewater treatments [15]. Among the existing techniques for MPs chemical identification Fourier Transform Infrared (FTIR) and Raman are the most commonly used techniques. μFTIR is capable of identifying MPs ranged between 20μm and 500μm, being the major drawback of this method that cannot identify particles<20μm [16]. On the other hand, because the laser beam in Raman spectroscopy is smaller than in FTIR, Raman spectroscopy can identify microplastics as small as a few micrometers. As an alternative, thermal degradation method based on a gas chromatographic mass spectrometer coupled with pyrolysis is established as a recognized method for qualitative and quantitative analyses of polymers capable of quantifying both MPs and NPs. During the sampling process, the choice of the used mesh size already limits the MNP size characterization and identification capacity of our methodology, with 20μm being the smallest screening mesh size mainly used until now [17]. As an example, Ben-David et al. [18] observed that including a finer mesh size of 0.45μm during the sampling method doubled the number of MPs found in the tertiary treatment effluents [18], showing the need of developing new sampling methods capable of capturing small MNPs. Polymeric Microfiltration (MF) and Ultrafiltration (UF) membranes can contribute to overcoming these limitations, by implementing them not only as a tertiary treatment, but also as a sampling method.
Polymeric Microfiltration and Ultrafiltration Membranes
In MF and UF membranes pressure is applied to overcome the
hydraulic resistance of the membrane for the mass transfer. MF
membranes work in a pore size range between 0.1-10mm, which
means that they require a relatively low operating pressure (0.1-
2 bar) and UF membranes have a pore size between 0.01-0.1mm,
operating at pressures between 2-6 bar. The small pore sizes of
these membranes make them viable for the separation of small
MNPs (<20μm) [19,20]. The separation of these micropollutants
can be conducted with different purposes such as:
A. Water purification: undesired MNP pollutants are removed
from wastewaters by implementing MF and UF membranes as
a tertiary treatment in WWTPs.
B. Concentration: small size MNPs can be present in water
environmental samples in low concentrations, making their
detection and identification difficult. A concentration step
would be required, where the solvent (water) is removed
retaining the desired compound (MNPs) in the concentrate
part.
The process performance of membrane separation processes is evaluated in terms of their selectivity, represented by the rejection coefficient (R, %), and the permeate flux (J, L/m2h) calculated with the following equations (Eq. 1 and Eq. 2):
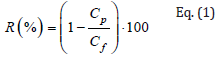
where Cp and Cf (g/L) are the concentrations of permeate and feed solutions, respectively
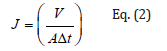
where V (L) is the volume of permeated water, A (m2) is the membrane area, and Δt is the permeation time at 25 °C temperature. Kook et al. [21] analyzed the removal efficiency and behavior of MNPs (PS and PE; 0,1 and 1.0μm) in membrane-based wastewater treatment, comparing polymeric and ceramic membranes. They observed that 1.0μm PS microplastic was successfully removed, obtaining 99.6% removal by all membranes, although they observed a slightly lower retention of over 96.0% for the 0.1μm PS microplastics. This was an expected result since the evaluated membrane had a pore size around 0.1μm. In order to improve the selectivity of the membranes towards the MNPs, other studies have been focused on preparing tailor made membranes for such purpose. Wang et al. [22] studied the removal efficiency of modified electro- spun membranes with controlled surface charge towards three different sizes of PS nanospheres (500nm, 100nm, 50nm). They observed that the positively charged membranes boosted the retention rate of the PS nanosphere to almost 100% due to the electrostatic interaction occurring between the charged membrane and the Nano plastic. Wan et al. [23] also prepared nanofibrous membranes to remove NPs in a gravity-driven membrane filtration process. The three types of studied NPs (range 107-1450nm) were successfully removed (92%) using the nanofibrous membranes in a gravity-driven mode, an energy-saving method because the required pressure is lower than the operating pressure of UF membranes. In addition, they showed that when diameter of NPs is bigger than the diameter of the membrane pore size, the NPs were rejected by the membrane pores via size- exclusive effect.
However, when diameter of the NPs is smaller than the diameter of the membrane pores, the NPs were adsorbed in the nanofibrous membranes by electrostatic attraction and hydrophobic interactions. During the filtration process, fouling remains one of the main issues that directly affect the membrane process performance in terms of reducing the permeate flux, changing the membrane selectivity or permeate quality, and increasing the membrane maintenance and replacement costs [24]. The fouling mechanism in the membrane is determined by the ratio of the foulant size (df) to membrane pore size (dpore) as following: (i) if dfddpore, the foulant can be adsorbed onto the membrane surface or dpore walls, or pass through the membrane; (ii) if df=dpore, the foulant tend to form a gel/cake layer or block the external or internal membrane pores; and (iii) if df>dpore, the foulant typically blocks the membrane surface pores or forms gel/cake layers at the membrane surface [24]. Enfrin et al. [25] studied the process performance of UF (PES; 30kDa) membranes towards a broad size distribution plastic particle from 12.5 to 689.6nm that were extracted from a cosmetic product used for facial scrub. They observed that during the filtration experiments, the water flux declines 38% due to the hydrophobic interactions between the NPs and the membrane, causing the adsorption of the NPs onto the pores and surface of the membrane. More specifically, they assumed that the fouling occurs first by intermediate pore blocking before all the pores get covered and then, the NPs accumulate forming a cake layer on the whole membrane surface. This kind of work is crucial to develop appropriate filtration and cleaning procedures, enhancing the membrane process performance. Therefore, further research on understanding the membrane fouling mechanism by MNPS is highly important.
Conclusion
MNPs are an emerging environmental challenge that has gained an outstanding interest in the past few years increasing the research effort to understand their identification and quantification, fate, impact as well as mitigation strategies. Membrane technology is a mature technology capable of eliminating small MNPs (<20μm) efficiently. However, research studies related to this application are scarce. This might be due to the lack of standardized processes and to the lack of efficient quantification and sampling methods for small MNPs that hinder a real evaluation of treatment technologies such as membrane technology. Further research on implementing membrane technology for the concentration and removal of small MNPs, gaining a deep understanding of the separation and fouling mechanism of these micropollutant should be encouraged.
Acknowledgement
The authors would like to thank the projects nano CLEAN, grant PID2019-111519RA-I00 funded by MCIN/ AEI/10.13039/501100011033 and μnano CARE, grant RTC2019- 007261-5 funded by MCIN/AEI/10.13039/501100011033 for their financial support.
References
- (2021) Plastics Europe Market Research Group (PEMRG) and Conversio Market & Strategy GmbH.
- Lambert S, Wagner M (2016) Characterisation of nanoplastics during the degradation of polystyrene. Chemosphere 145: 265-268.
- Gigault J, ter Halle A, Baudrimont M, Pascal P, Gauffre F, et al. (2018) Current opinion: What is a nanoplastic? Environ Pollut 235: 1030-1034.
- Hanif MA, Ibrahim N, Dahalan FA, Md Ali UF, Hasan M, et al. (2022) Microplastics and nanoplastics: Recent literature studies and patents on their removal from aqueous environment. Sci Total Environ 810.
- (2018) Plastics and the circular economy. A STAP Doc.
- Li J, Liu H, Chen JP (2018) Microplastics in freshwater systems: A review on occurrence, environmental effects, and methods for microplastics detection. Water Res 137: 362-374.
- Carr SA, Liu J, Tesoro AG (2016) Transport and fate of microplastic particles in wastewater treatment plants. Water Res 91 174-182.
- Murphy F, Ewins C, Carbonnier F, Quinn B (2016) Wastewater treatment works (WwTW) as a source of microplastics in the aquatic environment. Environ Sci Technol 50(11): 5800-5808.
- Talvitie J, Mikola A, Setälä O, Heinonen M, Koistinen A (2017) How well is microlitter purified from wastewater? - A detailed study on the stepwise removal of microlitter in a tertiary level wastewater treatment plant. Water Res 109: 164-172.
- Hsieh L, He L, Zhang M, Lv W, Yang K, et al. (2022) Addition of biochar as thin preamble layer into sand filtration columns could improve the microplastics removal from water. Water Res 221: 118783.
- Li B, Zhao J, Ge W, Li W, Yuan H (2022) Coagulation-flocculation performance and floc properties for microplastics removal by magnesium hydroxide and PAM. J Environ Chem Eng 10(2): 107263.
- Bayo J, López-Castellanos J, Olmos S, (2020) Membrane bioreactor and rapid sand filtration for the removal of microplastics in an urban wastewater treatment plant. Mar Pollut Bull 156: 111211.
- Luo M, Wang Z, Fang S, Song B, Cao P, et al. (2022) Removal and toxic forecast of microplastics treated by electrocoagulation: Influence of dissolved organic matter. Chemosphere 308(1): 136309.
- Pasanen F, Fuller RO, Maya F (2022) Fast and simultaneous removal of microplastics and plastic-derived endocrine disruptors using a magnetic ZIF-8 nanocomposite. Chem Eng J.
- Vighi M, Bayo J, Fernández-Piñas F, Gago J, Gómez M, et al. (2021) Micro and Nano-plastics in the environment: research priorities for the near future. Rev Environ Contam Toxicol 257: 163-218.
- Samanta P, Dey S, Kundu D, Dutta D, Jambulkar R, et al. (2022) An insight on sampling, identification, quantification and characteristics of microplastics in solid wastes. Trends Environ Anal Chem 36: e00181.
- Talvitie J, Mikola A, Koistinen A, Setälä O (2017) Solutions to microplastic pollution-Removal of microplastics from wastewater effluent with advanced wastewater treatment technologies. Water Res 123: 401-407.
- Ben-David EA, Habibi M, Haddad E, Hasanin M, Angel DL, et al. (2021) Microplastic distributions in a domestic wastewater treatment plant: Removal efficiency, seasonal variation and influence of sampling technique. Sci Total Environ 752: 141880.
- Lee A, Elam JW, Darling SB (2016) Membrane materials for water purification: Design, development, and application. Environ Sci Water Res Technol 2: 17-42.
- Ismail AF, Goh PS (2015) Microfiltration membrane. In: Kobayashi S, Müllen K (Eds.), Encycl Polym Nanomater, Springer Berlin Heidelberg, Berlin, Germany, pp. 1250-1255.
- Kook H, Park C (2022) Engineered approaches to facile identification of tiny microplastics in polymeric and ceramic membrane filtrations for wastewater treatment. Membranes (Basel)12(6): 565.
- Wang R, Zhang L, Chen B, Zhu X (2020) Low-pressure driven electrospun membrane with tuned surface charge for efficient removal of polystyrene nanoplastics from water. J Memb Sci 614: 118470.
- Wan H, Shi K, Yi Z, Ding P, Zhuang L (2022) Removal of polystyrene nanoplastic beads using gravity-driven membrane filtration: Mechanisms and effects of water matrices. Chem Eng J 450(4): 138484.
- Wang J, Cahyadi A, Wu B, Pee W, Fane AG, et al. (2020) The roles of particles in enhancing membrane filtration: A review. J Membr Sci 595: 117570.
- Enfrin M, Lee J, Le-Clech P, Dumée LF (2020) Kinetic and mechanistic aspects of ultrafiltration membrane fouling by nano- and microplastics. J Memb Sci 601: 117890.
© 2022 Junkal Landaburu-Aguirre. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






