- Submissions

Full Text
Polymer Science: Peer Review Journal
Study on Effect of Fillers in Passenger Car Radial Tyre Tread Cap Compounds
Ajay C*, Samui BK, Gupta SD and Mukhopadhyay R
Hari Shankar Singhania Elastomer and Tyre Research Institute (Hasetri), India
*Corresponding author: Ajay C, Hari Shankar Singhania Elastomer and Tyre Research Institute (Hasetri), Mysuru-570016, India
Submission: September 14, 2022;Published: October 21, 2022

ISSN: 2770-6613 Volume4 Issue3
Abstract
Carbon Black is still very widely used in passenger car tyre treads for reinforcement. Furnace Blacks including Intermediate Super Abrasion Furnace (ISAF) and High Abrasion Furnace (HAF) blacks can be seen in use for Tyre Tread compounds for optimized performance by matching the required mileage, traction, and rolling resistance. In this study, the Passenger car radial tyre tread compound was used for study by varying the grade of filler series to optimize the lab scale properties and cost. The compounds were characterized concerning their flow properties, rheological properties, dispersion, dynamic mechanical properties, and abrasion.
Keywords: Rubber; Carbon black; Polymer; Viscosity; Abrasion resistance
Introduction
Rubber is widely used in day-to-day life and almost all engineering disciplines. Rubber without filler materials has low mechanical properties and is of no use. Fillers are the compounding ingredients added to rubber for reinforcement and volume enhancement. Fillers modify the physical properties of both unvulcanized and vulcanized rubbers. Carbon black is the most popular filler which is commercially available; it is known for its ability to enhance the strength properties. This reinforcing carbon black produces a partial immobilization of the chain segments of the polymer. The extent of restriction of the rubber chain again depends on the nature of carbon black. The modification of an elastomer by carbon black reinforcement and vulcanization generates a unique 3D viscoelastic network that transforms the soft elastomers into a strong elastic product. Particle size and structure are the two major characteristics of Carbon blacks. Spherical nodules are considered particles. Aggregates are the smallest dispersible units of carbon black. Carbon black aggregates break down during mixing. These aggregates can fracture and generate free radicals that readily react with polymer chains to increase bound rubber [1].
The particle size is inversely related to Surface area. There are many methods available to measure the surface area of the carbon blacks, among which iodine adsorption [2], nitrogen surface area, and Cetyl Tri Ammonium Bromide (CTAB) [3] are very popular. Structure of the Carbon black is given by dibutyl phthalate (DBP and crushed DBP Numbers. Carbon black incorporation into the rubber matrix involves the breakup of the carbon black pellets. As mixing continues, pellet fragments break up and the rubber penetrates the voids between the black aggregates. Small particle-size blacks are difficult to incorporate. High structure blacks contain more void volume to be penetrated by the rubber and thus take longer time to get incorporated, but once incorporated they disperse more rapidly than low-structured blacks [4]. Tyres under dynamic conditions are meant to be subject to repeated deformations. It can lead to the generation of considerable heat. The extent of heat generation governs the change in viscoelastic properties of the product which may lead to premature failure. The nature of the filler used for tread application plays a major role over here. The Tread compounds with high hysteresis can lead to better traction and at the same time higher rolling resistance [5].
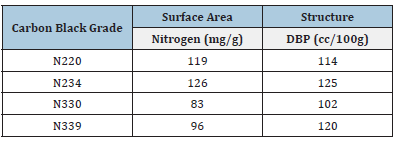
Generally, high surface area and high structure carbon blacks are associated with increased reinforcement in a rubber compound. Particle size impacts abrasion resistance, heat build-up (or resilience), tensile and tear strength, and flex resistance, whereas structure has more effect on modulus, hardness, and extrusion die swell [6]. The incorporation and effective dispersion of fillers into the rubber matrix are important for the performance of the product. The elastic part of a filler network depends majorly on its structure property, but still, the formation of the filler network and the process of aggregation are unclear [7]. The inferior rubber-tofiller interaction would affect the product life, processing difficulty, raw material, and energy wastage [8]. The selection of filler for tyre tread compound is important as the filler will be the rubber compound’s backbone for the required application. This work was carried out to identify a suitable filler for the Passenger tyre tread compound without compromising any of the key properties with optimum cost. Intermediate Super Abrasion Furnace (ISAF) black: N – 220 (in compound N1), N –234 (in compound N2) and High Abrasion Furnace (HAF) black: N – 330 (in compound N3), and N – 339 (in Compound N4) is subjected to this study. Typical properties of the Carbon black are given in below Table 1 [6].
Table 1:Typical properties of carbon black.
Experimental
Materials
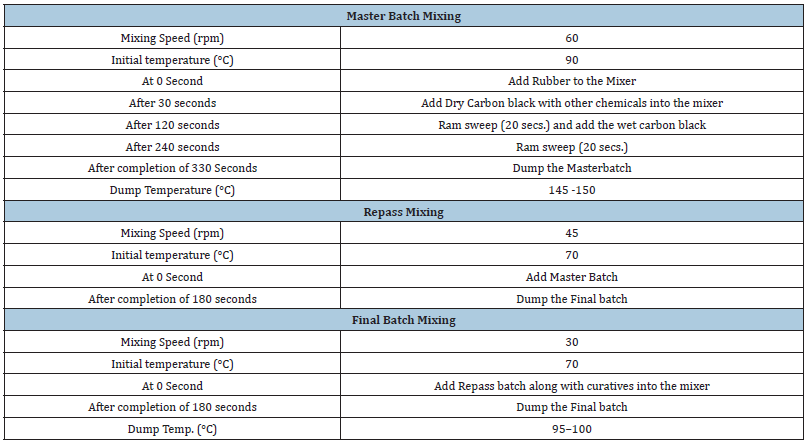
All the ingredients utilized in these studies were used as obtained. Styrene Butadiene Rubber (SBR 1502, 1723, and 1739) was supplied by Reliance Industries Pvt. Ltd., India. The other ingredients were N220, N234, N330, and N339 carbon black (Birla Carbon Black India Pvt. Ltd., India), Low PCA oil (Raj Petro, Chennai, India), zinc oxide (white seal; Zinc-o-India, India), stearic acid (Godrej Industries Ltd., India), N-phenyl-N’-(1,3-dimethylbutyl) p-phenylene-diamine (6PPD; National Organic Chemicals Industries Ltd., India), 2,2,4-trimethyl-1,2-dihydro-2,2,4-trimethyl quinoline (TMQ; National Organic Chemicals Industries Ltd., India), microcrystalline wax (Gujrat Paraffins Pvt. Ltd., India), N-cyclohexyl- 2-benzothiazole sulfenamide (CBS; National Organic Chemicals, India Ltd., India), soluble sulfur (Jain Chemicals Ltd., India), and diphenyl guanidine (DPG; National Organic Chemicals, India Ltd., India), N-(cyclohexylthio) phthalimide (PVI; National Organic Chemicals, India Ltd., India), used for the compound preparation. The formulations of the compounds are given in Table 2. The mixing of rubber compounds was carried out in a 1.5L volume Lab. Banbury mixer (Stewart Bowling, USA) with a 2-wing tangential rotor. The mixed batch was milled by using 2 roll-mill (Santosh Machinery, India). The mixing was carried out in three stages. The sequence of mixing is Master, Repass, and Final Batch. The mixing parameters including speed and dumb temperature remain kept uniform for all the compounds in different stages. Chamber temperature is maintained by using a temperature control unit. The details of the mixing sequence are given in Table 3. Molding of the test samples was carried out by using an 18’’ hydraulic operated curing press (Hind Hydraulics, India).
Table 2:Formulation of compounds.
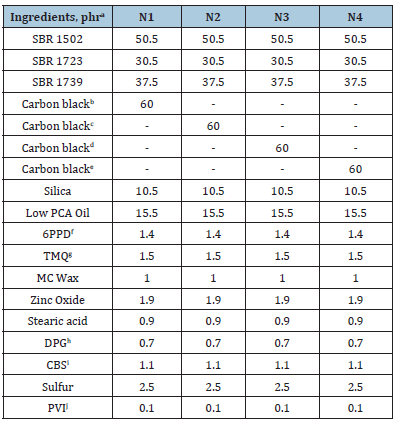
aphr, parts per hundred rubber by weight. bCarbon black grade N220 cCarbon black grade N234 dCarbon black grade N330 eCarbon black grade N339 fN,N’-diphenyl-p-phenylenediamine gN,N’-diphenyl-p-phenylenediamine>2, 2, 4-trimethyl-1,2-dihydro-2,2,4-trimethyl-quinoline hDiphenyl guanidine. iN-cyclohexyl-2-benzothiazolesulfenamide jN-(cyclo hexyl thio) phthalimide.
Table 3:Mixing sequence.
Characterization
Mooney viscosity was carried out on the compounds using Mooney Viscometer (Premier Mooney Viscometer from Alpha Technologies, USA) following the standard ASTM D1646. Mooney viscosity test was performed at the condition ML [1+4] @ 100 °C. Rheometric properties were studied by using Moving Die Rheometer (Premier MDR from Alpha Technologies, USA) as per the test standard ASTM D5289. The test was carried out at 160 °C for 30 minutes. Filler-Filler interaction (Payne Effect) was studied by using Rubber Process Analyzer (RPA 2000 from Alpha Technologies, USA) as per the test standard ASTM D8059. The Strain sweep (Payne Effect) test was carried out at a temperature of 50 °C, frequency of 1.66Hz and strain ranges from 0.1 to 100%. Cured strain sweep was studied by using Rubber Process Analyzer (RPA 2000 from Alpha Technologies, USA) as per the test standard ASTM D6601. The cure test was performed at 160 °C and then a strain sweep was performed at temperatures of 30 °C and 70 °C, frequency of 1.66Hz and strain ranges from 0.1 to 50%. Hardness (Shore-A) measurements were performed using Multi-Unit Hardness Tester (Gibitre Instruments, Italy) following ASTM D2240. The dumbbell specimens were stacked together to achieve the desired thickness for the measurement of hardness. Stress-strain properties were carried out by using Universal Testing Machine (Z010 Model, Zwick Roell, Germany) according to the standard ASTM D412. Die C Dumbbells were utilized for the study with a benchmark distance of 25±0.5mm and tested at a speed of 500mm/min with a grip-to-grip distance of 70mm. Dynamic Mechanical properties were carried out by using a Dynamic Mechanical Analyzer (DMA+1000 from ACOEM, France) according to ASTM D5992. A stabilized temperature sweep was performed at a temperature of 30 °C, 70 °C, and 100 °C, with Frequency at 11Hz, and strain of 5%. The abrasion study was carried out by using Din Abrasion Tester (Bareiss, Germany) by following the standard ASTM D5963. The Rotating mode test was performed with a speed of 40rpm and a load of 10N. Molding of samples for Hardness, Stress-strain, DMA, and Abrasion loss was carried out at 160 °C for 20 Minutes.
Results and Discussion
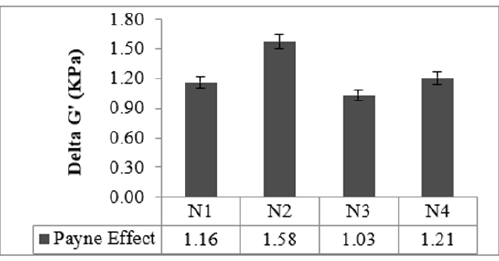
The Mooney viscosity of the studied compounds is given in Figure 1. Compound N2 showed the highest Mooney viscosity in both repassed and final batch compounds. N3 compound filled with low structure and low surface area carbon black showed the lowest Mooney viscosity. It was observed an increase in Mooney viscosity as the structure and surface area of carbon black increased. The flow properties of the compounds N2 and N4 are comparable. On increasing the surface area of the carbon black, the number of rubber chains restricts the movement of the carbon black and leads to an increase in Mooney viscosity [9]. The rheometric properties of the studied compounds are shown in Figure 2. From the rheometric study, Minimum torque (ML) was found to be highest for the high structure-filled compound N2 and the lowest for the low surface area black N3 compound. There was an increase in minimum torque value as the surface area and the structure of carbon black increased. Minimum torque reflects the measure of stiffness and viscosity of the uncured rubber compound. The Maximum Torque (MH) was observed as highest for high structure filled compound N2 and minimum for low surface area black compound N3, here the increase in MH was according to the increase in structure and surface area of the carbon black used. The torque value increases proportionately as the curing started and varied depending on the type of compound. Here, the curves for all the compounds were observed as plateau shaped. The Torque behavior for the compounds N2 and N4 are comparable. Scorch time or induction time (ts2), time for a fixed small rise above the ML, and the cure time (tC90) were observed to be comparable without any significant difference other than a reflection of torque values. The number of rubber chains entangling with the carbon black network increases as the surface area of the carbon black increases, and this leads to an increase in torque [10]. Filler-filler interaction (Payne Effect) of the studied compounds is given in Figure 3. Smaller the difference in shear storage modulus between the highest and lowest applied strain (delta G’) value higher the polymer filler interaction and the larger the delta G’ value, the higher the filler interaction. Here, polymer filler interaction was shown highest in low surface area black-filled compound N3, whereas the filler-filler interaction was found to be strong in High structure black-filled N2 compound. High-structure black would have been dispersed better by increasing the mixing time. Low strain testing deals with the linear viscoelastic region, the storage modulus G’, loss modulus G’’, and tan delta do not change with an increase in strain up to a limit, which is called critical strain. Hereafter G’, G’’, and tan delta decrease with increasing strain. Uncured mixed rubber compounds have shorter linear viscoelastic regions compared with raw elastomers. This is primarily due to the incorporation of carbon black and the establishment of an aggregate network, which is altered with raising strain [11].
Figure 1:Mooney viscosity.
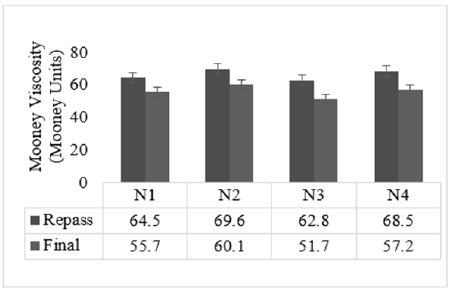
Figure 2:Rheometric properties.

Figure 3:Payne effect study.
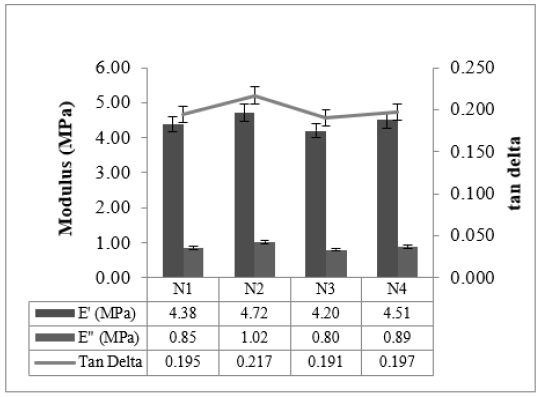
Cured strain Sweep of the studied compounds is given in Figures 4 & 5. The test gives an idea about the after-cure properties. Low surface area black-filled compound N3 showed good polymer filler interaction after crosslinking in both the sweep conditions @ 30 °C and @70 °C, whereas N2 displayed higher filler-filler interaction in both the sweeps. The test data agrees with the Payne effect test performed on an unvulcanized compound. The addition of fillers into the rubber matrix can form an extensive network and affect the mechanical behavior [11]. After cure, dynamic property measurements at low temperatures are important to relate to the product service conditions [12]. The stress-strain properties of the studied compounds are outlined in Figure 6. Stress @ 300% Elongation (300% Modulus) also known as the stiffness of the rubber compounds found to be highest for high structure black-filled compound N4 and lowest for high surface area blackfilled compound N3. The modulus increases while increasing the structure of the black. The modulus property of N2 and N4 are comparable. Tensile strength is found to be maximum for high surface area black filled N2 and minimum for low surface area N3 compound. The tensile strength increases while increasing the surface area of the black. Breaking Elongation was found to be highest in high-structure black-filled N3 compound, were as lowest for N4 compound. Breaking elongation decreases while increasing the structure of black. The compound with higher reinforcement better would be the wear characteristics [13]. As the surface area of the carbon blacks goes higher, the rubber chain entanglements become restricted, and breaking elongation becomes low [14]. The hardness properties of the studied compounds are given in Figure 7. Hardness is the best tool for quality control, was found to be highest for high structure black-filled compound N2 and lowest for high surface area black-filled N3 compound. The hardness properties of N2 and N4 are found to be comparable. Dynamic Mechanical Properties (@30 °C) of the studied compounds are given in Figure 8. The dynamic storage modulus (E’), dynamic loss modulus (E”), and loss factor (tan d) were measured by using a Dynamic Mechanical Analyzer. High surface area black-filled compound N2 showed the highest tan delta and low surface area black-filled compound N3 showed the lowest tan delta. Storage modulus at 30 °C gives an idea of the dry grip properties [15]. Modulus and tan delta increased as the structure of black increased. Dynamic Mechanical Properties (@70 °C) of the studied compounds are given in Figure 9. Low surface area black-filled compound N3 showed the lowest tan delta and high surface area black-filled compound N2 showed the highest tan delta. tan delta at 70 °C gives an idea of the rolling resistance properties [13]. tan delta increased as the structure of black increased like properties at 30 °C.
Figure 4:Cured strain sweep 30 °C.
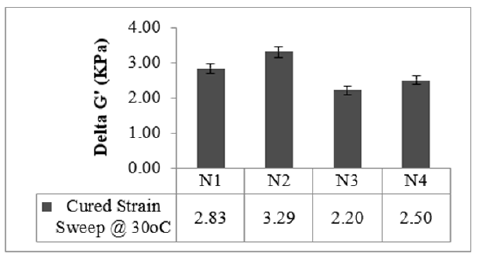
Figure 5:Cured strain sweep 70 °C.
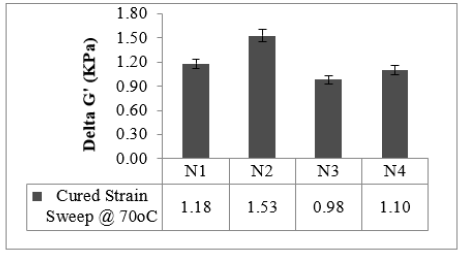
Figure 6:Stress-strain properties.
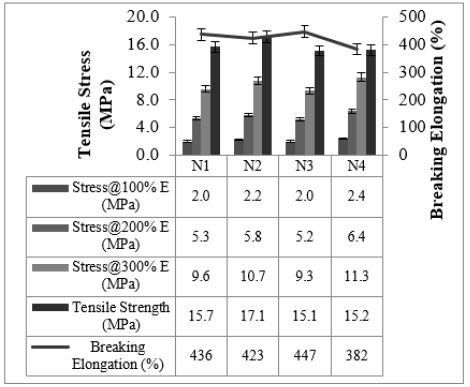
Figure 7:Hardness properties.
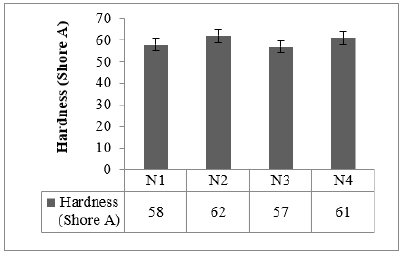
Figure 8:Dynamic mechanical properties @ 30 °C.
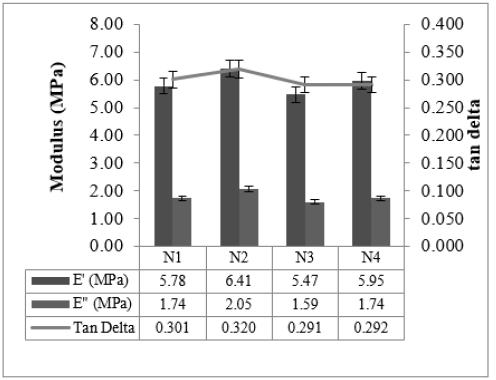
Figure 9:Dynamic mechanical properties @ 70 °C.
Dynamic Mechanical Properties (@100 °C) of the studied compounds are given in Figure 10. Low surface area black-filled compound N3 showed the lowest tan delta and high surface area black-filled compound N2 showed the highest tan delta. tan delta value at 100 °C gives an idea of the heat build-up properties [16]. tan delta value increased as the structure of black increased like properties at other temperatures. The abrasion properties of the studied compounds are given in Figure 11. High Structure black-filled compounds N2 and N4 showed the highest abrasion resistance and the low surface area black-filled compound N3 with least abrasion resistance. The abrasion properties of N2 and N4 are comparable. Rubber compounds filled with lower particle size and higher structure blacks showed better abrasion resistance [17].
Figure 10:Dynamic mechanical properties @ 100 °C.
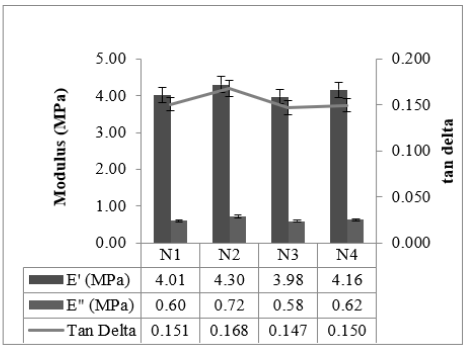
Figure 11:Abrasion properties.

Conclusion
Compound N2 filled with High structure high surface area Carbon black showed superior properties throughout the studies. Compounds N2 and N4 showed comparable performance in properties such as flow properties, torque response, hardness, modulus, and abrasion resistance. ISAF N234 black remains costlier (price/kg) when compared to all the other three fillers. Depending on the requirement and application, for optimum performance with properties by not compromising the cost N3 would be always a good substitute for the N2 compound.
Acknowledgment
The authors would like to thank the Management of Hari Shankar Singhania Elastomer and Tyre Research Institute for granting permission to publish this work.
References
- Garten VAK, Eppinger D, Weiss E (1956) Studies on abrasion and wear of rubber. The chemistry of carbon black and its effect on abrasion as determined by the National Bureau of Standards method. Rubber Chemistry and Technology 29(4): 1434-1444.
- Wiegand WB, Snyder JW (1931) Some properties of carbon black. I. adsorption. Rubber Chemistry and Technology 4(3): 407-416.
- Magee RW (1995) Evaluation of the external surface area of carbon black by nitrogen adsorption. Rubber Chemistry and Technology 68(4): 590-600.
- Medalia AI (1978) Effect of carbon black on dynamic properties of rubber vulcanizates. Rubber Chemistry and Technology 51(3): 437-523.
- Hess WM, Klamp WK (1983) Effects of carbon black and other compounding variables on tire rolling resistance and traction. Rubber Chemistry and Technology 56(2): 390-417.
- Gupta SD, Mukhopadhyay R, Baranwal KC, Bhowmick AK (2013) Reverse engineering of rubber products: concepts, tools, and techniques. CRC Press, US.
- Meier JG, Klippel M (2008) Carbon black networking in elastomers monitored by dynamic mechanical and dielectric spectroscopy. Macromol Mater Eng 293(1): 12-38.
- Ganesan L, Bhattacharyya P, Bhowmick AK (1995) Quantitative measurement of the dispersion of carbon black in rubber by an image processing technique. J of Appl Polym Sci 56(13): 1739-1747.
- Li ZH, Zhang J, Chen SJ (2008) Effects of carbon blacks with various structures on vulcanization and reinforcement of filled ethylene-propylene-diene rubber. eXPRESS Polymer Letters 2(10): 695-704.
- Litvinov VM, Steeman PAM (1999) EPDM-carbon black interactions and the reinforcement mechanisms. as studied by low-resolution 1H NMR. Macromolecules 32: 8476-8490.
- Payne AR, Whittaker RE (1971) Low strain dynamic properties of filled rubbers. Rubber Chemistry Technology 44(2): 440-478.
- (2019) Standard test method for rubber properties-measurement of cure and after-cure dynamic properties using a rotorless shear rheometer (ASTM D6601).
- sridharan H, Guha A, Bhattacharyya S, Bhowmick Ak, Mukhopadhyay R (2019) Effect of silica loading and coupling agent on wear and fatigue properties of a tread compound Rubber Chemistry and Technology 92(2): 326-349.
- Pöschl M, Vašina M, Zádrapa P, Měřínská D, Žaludek M (2020) Study of carbon black types in SBR rubber: mechanical and vibration damping properties. Materials 13(10): 2394.
- Warasitthinon N, Robertson CG (2018) Interpretation of the tanδ peak height for particle-filled rubber and polymer nanocomposites with relevance to tire tread performance balance. Rubber Chemistry and Technology 91(3): pp. 577-594.
- Ulmer JD, Chirico VE, Scott CE (1973) The effect of carbon black type on the dynamic properties of natural rubber. Rubber Chemistry and Technology 46(4): 897-926.
- Bolt TD, Dannenberg EM (1961) Effects of carbon black structure on tire tread wear. Rubber Chemistry and Technology 34(1): 43-56.
© 2022 Ajay C. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






