- Submissions

Full Text
Polymer Science: Peer Review Journal
Study of Microcapsules of Essential Oils: Drug Delivery and Particle Characterization
Lis Arias MJ1*, Repetto Baubin MA1, Escorza FG1, Rubio JP1, López A1 and Meng X2
1Department of surfactants and detergency Intexter, University of ESEIAAT (UPC), Spain
2Textile Technology Department, Shaoxing University, China
*Corresponding author: Manuel J Lis Arias, Department of surfactants and detergency Intexter, University of ESEIAAT (UPC), No. Barcelona, Spain
Submission: August 05, 2022;Published: September 07, 2022

ISSN: 2770-6613 Volume4 Issue2
Abstract
Essential Oils (EOs) are a substance composed of two phases. A liquid and volatile substance that has aromatic, antibacterial, antioxidant. The properties of EOs are not new to humanity. Indeed, these began to take a role from the first civilizations (Egyptian civilization). Where EOs used as preservatives and flavourings. Nowadays, the use of the EOs is the same. Avoid the growth of bacteria and viruses (theoretical) in food, the contact surface (chairs of the bus), tables, fabrics (clothes). During these last years, the industrial world took the relevance of the microencapsulation technique. This technique pretends to capture the EOs to reach two main objectives. First, contain all his active compounds into a microcapsule to prevent the degradation of the environment. Second, release control into the media, contact surface, or fabrics. Microencapsulation is a beneficial technology to improve fields of biomedicine and the food industry. Besides, the impregnation of these microcapsules on fabrics can develop a lot of new properties. Indeed, the antibacterial and antioxidant properties. The amount of these properties requires an accurate and meticulous methodology. The formation of the microcapsules is very complicated to explain from one point of view. Therefore, it is necessary to collect all the available data. Nevertheless, the research count with very sophisticated equipment, which can provide us with very accurate results. Nanosizer, Spectrophotometer, SEM.
The meaning of this research is to make sure if it is possible to control either the diffusion of the EOs and his chemist properties (diameter and morphology). Simple coacervation of microcapsules of β-cyclodextrin (B-CD) with lavender (L) is the technique that we will follow at the beginning of this research. These simple microcapsules will be impregnated in different fabrics: Polyester (PET), Cotton (COT), and Polyamide (PA). After his impregnation, we must wait until dry in the ambient and make the drug delivery. The complex coacervation is the principal object to study. In this part of the research, we will use Eco Tween 20 (TW) as a surfactant and some crosslinkers: Citric Acid (CA) and Tannic Acid (TA). Due to his addition, we will pay attention to his new chemist properties and behaviour in different fabrics. The drug delivery results will follow a mathematical procedure. This procedure, which will contrast with Korsmeyer-Peppas and Higuchi principles, will help us ascertain his diffusion behaviour.
Keywords:Essential oil; Microcapsules; Complex coacervation; Drug delivery; Fabrics; Surfactants; Crosslinkers; Korsmeyer-peppas; Higuchi
Abbreviations: EOs: Essential Oils; PET: Polyester; COT: Cotton; PA: Polyamide; CA: Citric Acid; TA: Tannic Acid; CMC: Critical Micelle Concentration; HLB: Hydrophile-Lipophile Balance; AC: Active Compound
Introduction
Nowadays, the microencapsulation of EOs is being widely common [1]. The capacities of this technology are extremely useful in the fields of biomedicine and the food industry [2]. EOs are characterized by being a liquid and volatile substance that has aromatic behavior. These EOs have some special properties that can be extremely useful [3]. For instance, his antibacterial and antioxidant properties [4,5]. The techniques of encapsulation are widely different. However, we can distinguish two main groups: Chemical and physics processes. The chemical processes are most interesting due to their efficiency [6,7]. Coacervation of simple and complex systems is the technique that we are going to carry out. Besides, the formation of microcapsules is a spontaneous and irreversible process. Attributable to his Gibbs free energy, which is a negative number [8]. The microcapsules of lavender in other articles are formed by using this technique to improve stability and uniformity. There are many options to stabilize the microcapsules. Simple and complex coacervation [9], following (Figures 1 & 2). The coacervate is made up of a polymeric solute separated in the form of small liquid droplets. This arrangement of the dispersed insoluble particles suspended or emulsified in a hydrocolloid medium and the coacervate form incipient capsules that will become final capsules through their solidification by gelification [10].
Figure 1:Complex coacervation process [7].
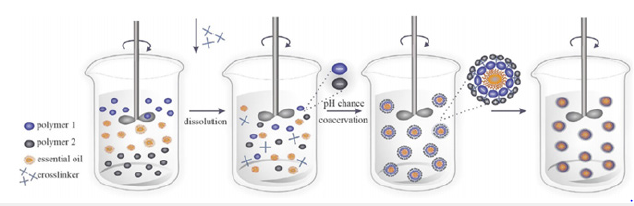
Figure 2:Simple coacervation process [10].
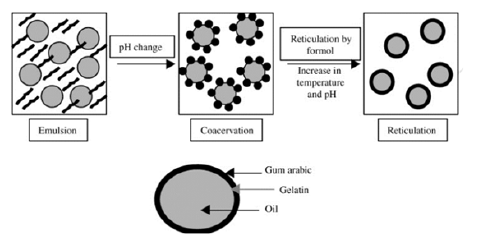
The coacervation depends mainly on the physicochemical characteristics of the polymer and the core to be coated. First, the development of the phase separation is induced by the slow addition of a non-solvent to a solution of the coating-forming polymer, containing suspended material to be encapsulated. Second, the non-soluble is understood as that which is miscible with the polymer-solvent and in which the polymer is insoluble. As the non-solvent is added, we cause the in solubilization of the polymer, which is deposited around the particles present in suspension. Third, the termination of the process can be heating, desolvation, or crosslinking techniques to support the microcapsules [5]. The core material must be insoluble in the coacervation medium and must be compatible with the polymer in the container. The development of EO emulsification in an aqueous solution contains two phases (a polysaccharide and a protein). In the first step, we will place ourselves at a temperature higher than that of gelling and a pH above the isoelectric point of the protein to favor a good movement of the AC and the coating polymer [11]. In the second step, the separation of the liquid phase from the insoluble polymerrich phase because of electrostatic attraction between oppositely charged polymers caused by lowering the pH of the solution below the isoelectric point of the protein. The third step consists of the formation of walls due to the deposition of the polymer-rich phase around the hydrophobic droplets, followed by a controlled cooling below the gelation temperature. In the last step, the hardening of the wall of the microcapsules is achieved by the addition of crosslinking agents.
Layer by layer. Consist of the creation of a multilayer system. This sequential deposition of polyelectrolytes with an opposite electrical charge is the main principle to create it [12]. The process is described in Figure 3. The technique pretends to control the chemical behavior of the microcapsules. This field is currently studied by the principles of Langmuir-Blodgett [13]. The research field of surface chemistry. Therefore, the superficial monolayer is responsible for the chemical and physical properties. At the same time, the remaining one is mainly involved in the stabilization of the overall structure. The surfactant agent (Span 80, Tween 20, SDS…) stabilizes the microcapsule. The chemical behavior of this agent improves the solubility in hydrophilic substances and avoids drug recrystallization. The surfactant has both behaviors: hydrophilic and hydrophobic part. Commonly named amphiphilic nature. Moreover, the surface tension is reduced when are absorbed in a specific interface and they can be self-assembled or self-associate (allows micelle formation) [7]. The micelle is formed at a specific concentration. Critical Micelle Concentration (CMC). The control of this parameter affects the emulsification and coacervation process. The value of Hydrophile-Lipophile Balance (HLB) will help us to choose the appropriate emulsifier (or surfactant agent) [14]. This measure is a relative parameter between hydrophobicity and lipophilicity. Lipophilic character is represented by a low value of HLB (below 9.0) and a high value of HLB (above 11.0) for hydrophobic. In essential, the emulsifier used in the encapsulation process determines sizes, morphology, encapsulation efficiency, control release.
Figure 3:Layer by layer process [12]..
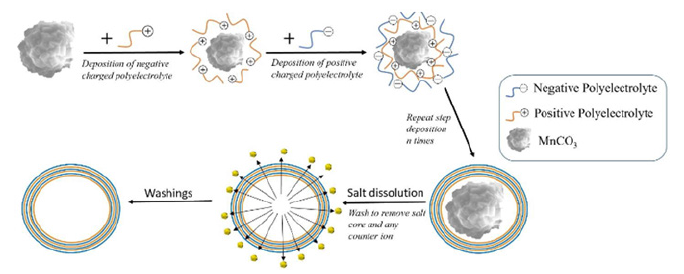
Crosslinkers (CA, TA, Trypolyphosphate…) modify the chemical structure of the microcapsule. The interaction between the polymer site (encapsulation agent) and crosslinkers affects its chemical and physical properties. For instance, mechanical resistance, permeability, chemical, and thermal stability, impregnation on a specific fabric, and his release in the media [7,15,16]. The research pretends to study the different interactions of each crosslinker (CA and TA). The CA is a short chain of three carboxylic acids, which can be a problem when the microcapsule reaches a determined size. On the contrary, the TA is a large organic chain that can provide to the microcapsule a huge efficiency capture of EO and size. The best way to study either morphology of microcapsules or finishing fabric fibers is with an SEM. The research pretends to study both his morphology and his drug delivery. Due to the antibacterial properties of the EOs, different types of fabrics like gauze, masks, and hospital gowns are impregnated with microcapsules to prevent the transmission of bacteria [17,18]. The two main objectives for this type of application are control of the release of the Active Compound (AC) in our case it is lavender and controls the morphology. In this research, we will study the results of simple microcapsules (B-CD with L) and complex (using surfactants and crosslinkers at different mass ratios) to see differences in either in the release of the AC and the morphology. Besides, we will study these microcapsules in three different fabrics (PET, COT, and PA) [19].
Complex microencapsulation: First of all, we must create the micelle system before using the B-CD. TW will help us to obtain a micelle, that micelle is pre-treatment before the formation of the microcapsule. This pre-treatment will improve stability and promote size uniformity. To create the micelle, we must dissolve the surfactant TW into a deionized water base. This dissolution must be at 10% (w/w), it can take 30min of stirring until reaches a clear solution. At once, we have both the TW dissolution and the EO of lavender we can start mixing L with the TW in three different mass ratios 1:1, 1:2, and 1:3 (L-TW). The mixing must take 30min unless to give time enough for the creation of the micelle.
The creation of the micelle is concluded. Now, we must pay attention to the microencapsulation process. The ratio between L and B-CD must be the same as in the simple microencapsulation process 1:1. To complete the microencapsulation process we must stir and wait for 4 hours, to be sure that all the B-CD is set on the surface of the micelles. The crosslinkers need to be solved into a 10% (w/w) water base, both CA and TA. In the case of TA, it can take more time to reach a clear solution. After the creation of these two dissolutions, we must start to mix at mass ratios 1:1, 1:2, and 1:3 (BCD and Crosslinkers), for each mass ratio either of TW or different crosslinker. For example, mass ratio 1:1 (L with TW) and 1:1 (B-CD and CA) will be named as L-TW-11-CA-11. To simplify LTW11CA11. And the series will continue as LTW11CA12, LTW11CA13, swap surfactant ratio, LTW12CA11, …, LTW13CA13. And the same for TA (Scheme 2)
Materials and Methods
Materials
Lavender essential oil (L) was purchased from Terpenic Lab., La Garriga, Spain. β-cyclodextrin (B-CD) was purchased from Grupo Carinsa., Barcelona, Spain. Tannic Acid (TA, CAS: 1401-55-4) was purchased from Alfa Aesar., Karlsruhe, Germany. Citric Acid (AC) was purchased from Panreac., Barcelona, Spain. Eco Tween 20 (TW) was supplied by Croda for research purpose., Barcelona, Spain.
Scheme 1:Simple microencapsulation.
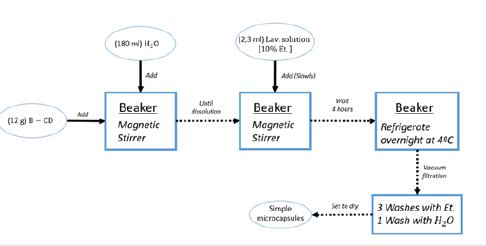
Simple microencapsulation: First, take B-CD (12g) and add into deionized water (180ml), and stir until dissolved. Ambient temperature. Second, add slowly and stir 2,3mL of the solution of lavender EO (ethanol 10% w/w) for 4 hours and refrigerate overnight at 4 ºC. This will set the microcapsules at the bottom of the beaker. Third, vacuum filtration (büchner), wash three times with absolute alcohol and once with deionized water. Fourth, set to dry (Scheme 1).
Complex microencapsulation: First of all, we must create the micelle system before using the B-CD. TW will help us to obtain a micelle, that micelle is pre-treatment before the formation of the microcapsule. This pre-treatment will improve stability and promote size uniformity. To create the micelle, we must dissolve the surfactant TW into a deionized water base. This dissolution must be at 10% (w/w), it can take 30min of stirring until reaches a clear solution. At once, we have both the TW dissolution and the EO of lavender we can start mixing L with the TW in three different mass ratios 1:1, 1:2, and 1:3 (L-TW). The mixing must take 30min unless to give time enough for the creation of the micelle.
The creation of the micelle is concluded. Now, we must pay attention to the microencapsulation process. The ratio between L and B-CD must be the same as in the simple microencapsulation process 1:1. To complete the microencapsulation process we must stir and wait for 4 hours, to be sure that all the B-CD is set on the surface of the micelles. The crosslinkers need to be solved into a 10% (w/w) water base, both CA and TA. In the case of TA, it can take more time to reach a clear solution. After the creation of these two dissolutions, we must start to mix at mass ratios 1:1, 1:2, and 1:3 (BCD and Crosslinkers), for each mass ratio either of TW or different crosslinker. For example, mass ratio 1:1 (L with TW) and 1:1 (B-CD and CA) will be named as L-TW-11-CA-11. To simplify LTW11CA11. And the series will continue as LTW11CA12, LTW11CA13, swap surfactant ratio, LTW12CA11, …, LTW13CA13. And the same for TA (Scheme 2).
Scheme 2:Complex microencapsulation.

Particle size
The particle size of the microcapsules was determined by using a Nanosizer S. (Malvern Instruments, UK). To determine the particle size, the samples were introduced into the cuvettes (DTS0012). Nevertheless, the use of electronic (JSM-5610, JEOL) and optic microscope (Olympus Bx43F) is required. This will help the research to prove with images both the formation of microcapsules and the differences between his size and layers
Application of microcapsules on fabrics
Fabric impregnation: The main objective of fabric impregnation is to attach the microcapsules to the fabric fibers. The technique used has two steps. First, immersion of the fabric into the microcapsule’s solution. And second, extenuation part. When the microcapsules start to set and attached to the fabric fibers (PADBATCH). We have to impregnate every sample with three different fabrics (PET, COT, PA). The time of the set of the microcapsules must be for all the samples the same, about 3min at ambient temperature. To finish, set to dry at ambient temperature.
Characterization of microcapsules on fabrics: On the other hand, morphology was taken by using an SEM (JSM-5610, JEOL) The samples were taken from impregnated fabrics. First, the sample fabrics were set on an electron platform with conductive glue. Second, the sample was sputtered with a gold surface to make conductive the surface of samples and to allow the free circulation of electrons.
Drug delivery:In this process, we will determine the capacity of desorption of the AC in aqueous media. First, cut a piece of impregnated fabric and measure his weight. Take a beaker and put it inside 20ml of deionized water per gram of fabric. Start the thermostatic bath and set the temperature at 37 ºC. Wait until reaching the desired temperature both in the bath and inside of the beaker. At once, prepare 34 test tubes of 1,5ml capacity. At the same time that you put the impregnated fabric inside the beaker, you have to take a sample of 1ml from the water media, this will be the sample at time 1 second and refill the beaker with 1ml of deionized water with the same temperature. After this first pickup, you have to continue taking samples every minute from 1min to 30min and repeat every hour from 1h to 3h. These samples will be analyzed in a spectrophotometer UV-Visible (Shimadzu UV-2401 PC) to determine his concentration of lavender.
Evaluation of drug delivery results: The capsules aim to isolate the nucleus until the moment of release. This release occurs through several different mechanisms, by diffusion, mechanical breakage, by a modification of the pH, etc [20]. Diffusion can come from polymers, where prior knowledge of the mechanism of solute diffusion through the polymeric material is required. There are also systems controlled by release from diffusion, where an amount of active reaches a particular area that is controlled by the phenomenon of diffusion through the molecular structure of the polymer [21]. Normally, the most common system is a combination of the two methods, the first where the nucleus is contained within a homogeneous, non-porous and thin membrane, and where it can be dissolved or dispersed in this polymeric wall, where the release process takes place [22].
Within the mechanism of release by diffusion there are different mathematical models that determine the type of diffusion given, whether it can be Fickiniana or anomalous. All the empirical mathematical models to be developed below are based on Fick’s first and second laws. There are different equations that determine kinetics in a system and based on this the release of the active ingredient can be studied. One of the best-known laws is the Power lab Equation, where it is proposed that the diffusion mechanism frequently deviates from a Fickinian diffusion and follows an anomalous behavior. Therefore, it is used to measure the effects of fickinian diffusion and relaxation of polymeric systems.
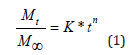
Mt refers to the amount of active substance released at the time, M∞ to the maximum amount of the drug released in an infinite time or equilibrium, k is the constant rate of drug release, n is a release exponent that depends on the geometry of the system
When the exponent n has a value of 1, the release rate does not depend on time, therefore it corresponds to a release rate of order cero. Zero-order release is known as the process of relaxation of molecules that occurs when absorbing water into the system. This water that enters the system is responsible for lowering the vitreous temperature and facilitating the mobility of molecules and their expansion in volume. This may be due when n=0.5 where the release occurs by diffusion or n=1 when the active substance is released by water absorption [23]. Another release model of the active substance is based on the Higuchi model. This method has been updated, since at first it was based on the fact that the release came from a perfect plane, while later it was intended to study the release in homogeneous and granular matrices. Therefore, the equation can be applied when the active substance is uniformly dispersed [22]. When the nucleus is dispersed, it acts by diffusion to the surface. This model is known as the following equation:
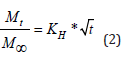
Mt is the absolute amount of active substance released in time
t, M∞ the total amount of active substance released in infinite
time, t is time, kH is the constant of dissolution/velocity of Higuchi
 , where D is the diffusion coefficient, Cs the
solubility of the active substance y C0 the initial concentration of the
active substance. The Higuchi equation is based on a pseudo-steady
state and cannot be applied in real controlled-release systems.
, where D is the diffusion coefficient, Cs the
solubility of the active substance y C0 the initial concentration of the
active substance. The Higuchi equation is based on a pseudo-steady
state and cannot be applied in real controlled-release systems.
The Higuchi equation has some limitations, but its simplicity makes it widely used today. The initial concentration of the active substance in the system is much higher than the solubility of the active ingredient, in order to justify the pseudo-steady state. The edge effects and swelling of the Prepolymer must be negligible, because it is based on a one-dimensional diffusion. The drug used is in a fine state where the particles are much smaller in diameter than in thickness [23]. In addition, a proportionality between the fractional quantity of the released active ingredient and the square root of time shown in equation 2 can be derived from an exact solution of Fick’s second law. Therefore, it can be said that the diffusion of the active substance can be studied as a flat surface in short release times as shown in equation 3:
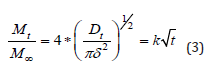
Where D is the diffusion coefficient, δ is the thickness of thin films under perfect conditions. Therefore, a ratio between the fraction of the active substance released and the square root of time can also be based on these circumstances which are different from those studied by Higuchi from the derivation of his classical equation [5,11,24].
Results and Discussion
Characterization of microcapsules
State of aggregation:This part aims to observe the state of aggregation of the emulsion of complex microcapsules. The samples of microcapsules formed by CA demonstrate that it is an emulsion because of its white color. This phenomenon is known as the Tyndall effect. When light through a sample that contains particles that are not diluted in the dissolvent (e.g., water), shows a color. In this case, it is white because his particles in suspension (microcapsules) reflects all the wavelength wave. However, the suspension of these microcapsules tends to precipitate because of their huge mass concerning water media. We assume that the complex microcapsules have uniformity and do not tend to create huge microcapsules that could precipitate and get stuck on the bottom. Even, the series that contain more mass ratio of surfactant and crosslinker does not affect the nature of the emulsion (Photographs 1-3).
Photograph 11Series LTW11CA.

Photograph 2:Series LTW12CA.

Photograph 3:Series LTW13CA.

Photograph 4:Series LTW11TA.

On the contrary, the behavior of microcapsules formed by TA is widely different. The first series of emulsions just have 2 phases. The same number and behavior (but, in this case, it is grey) as in the emulsion series of CA. However, the series LTW12TA and LTW13TA presented in Photographs 4-6, appear in two more phases. The first series of emulsions have the same behavior as in the case of CA [25]. But, in the series, LTW12TA appears a new phase of brown color. This phase is a result of a huge number of microcapsules aggregates that conforms to a very sticky phase that is attached to the bottom and the walls of the flask. The series LTW13TA is a phase state, in which we can only distinguish two-phase. The phase contains microcapsules suspended in water. These microcapsules are the tiniest microcapsules that were not aggregated into the huge sticky phase that now is very attached to the bottom and the walls. Therefore, the samples of the series of LTW12TA and LTW13TA will not continue the research because of his very complex state of aggregation.
Photograph 5:Series LTW12TA.

Photograph 6:Series LTW13TA.

Particle size: This part of the results will pretend to contrast the particle size between simple and complex microcapsules. Table 1 contains all the sizes of the microcapsules: Graph 1 shows the evolution of the sizes of the microcapsules. At first look, it is possible to see a growing evolution in both cases. The CA has the biggest size with 3380nm of diameter, the second it follows with 3170nm of TA. The size evolution of the complex microcapsules is very similar. Both curves have three phases: first, small values with a positive growth (higher in the case of TA); second, reach the maximum value; third, moderate values with negative growth.
Graph 1:Evolution of size front mass ratios increase.
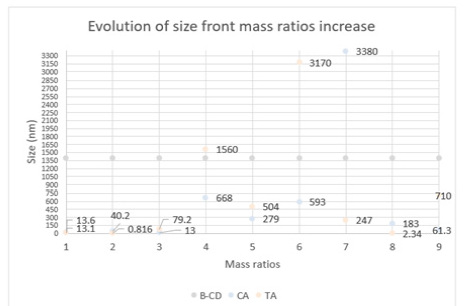
Table 1: Diffusional exponent and mechanism release from various non-swellable controlled release systems.
The curves describe perfectly the systems that we were explaining before in the part of the state of aggregation. Both complex microcapsules grow until a critical point, in which, the behavior starts to envelop. The CA system as we described before, has in all of its series an emulsion state where the microcapsules are in suspension. The graph shows that the microcapsules of CA after the critical point have more big diameters than in the case of TA. Despite the addition of more crosslinkers, the CA microcapsules started to create and distribute this additional crosslinker into new moderate microcapsules sizes. On the contrary, the TA microcapsules tend to aggregate until reaching an enormous aggregation of microcapsules. Because of this fact, the only microcapsules present in the evolution of size are the tiniest. The microcapsules avoid this enormous aggregation. The main reason for this different behavior is found in the chemical structure. The CA is a very tiny carbon chain that allows great versatility and displacement. On the contrary, the TA is a very large carbon chain that tends to be very slow and messy. As we can observe in Figure 4, the lack of surfactants and crosslinkers generates a chaotic system. This system shows a huge number of different diameter sizes, differences in wall-width, encapsulation of AC and water (microcapsule of 28,43um) … Because of his lack of uniformity and stability, this is not a desirable system to study. The use of surfactants and crosslinkers are the main reason to prevent this type of system, which cannot be considered in a drug delivery study. The efficiency of encapsulation between TA and CA is quite different. The TA microcapsules present a clean background and a thin-width wall. On the contrary, the CA microcapsules have a bigwidth wall with a messy background. The CA needs more units to build a single microcapsule (Figures 5-7).
Figure 4:Simple microcapsules extracted from microscope.
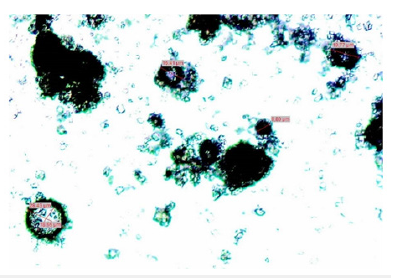
Figure 5:Microcapsule of LTW11CA11 extracted from a microscope, 8X..
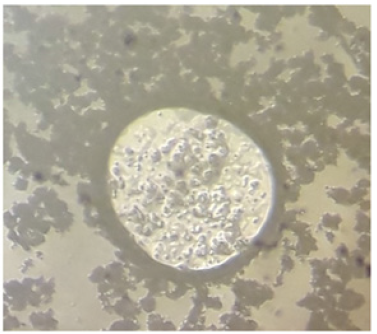
Figure 6:Microcapsule of LTW11TA11 extracted from a microscope, 25X.
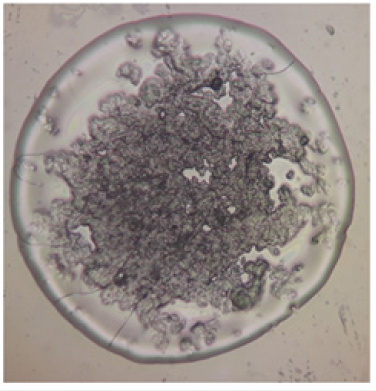
Figure 7:PET fabric impregnated with LTW12CA11 extracted from SEM.
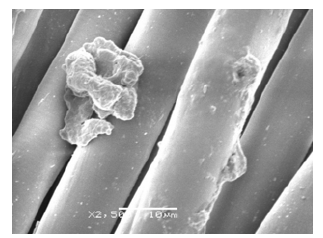
Morphology: TA microcapsules have very strong adhesion in all the fabrics. The microcapsules as we mentioned before, tend to aggregate into a huge microcapsule. This fact is perfectly described in Figures 8-10. Besides, impregnation has changed completely the physical and chemical properties of all the fabrics. Figure 8 shows an attachment of three fibers. On the contrary, the CA microcapsules were correctly impregnated in two of three fabrics (just only in PET and PA). COT did not present any microcapsule in the fabrics (Figure 11).
Figure 8:PET fabric impregnated with LTW12CA11 extracted from SEM.
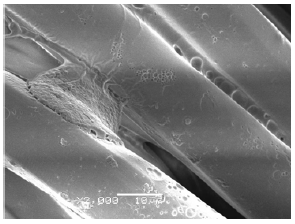
Figure 9:COT fabric impregnated with LTW11TA12 extracted from SEM.

Figure 10:PA fabric impregnated with LTW11TA12 extracted from SEM.

Figure 11:PA fabric impregnated with LTW12CA11 extracted from SEM.

Drug delivery
Microcapsules of CA
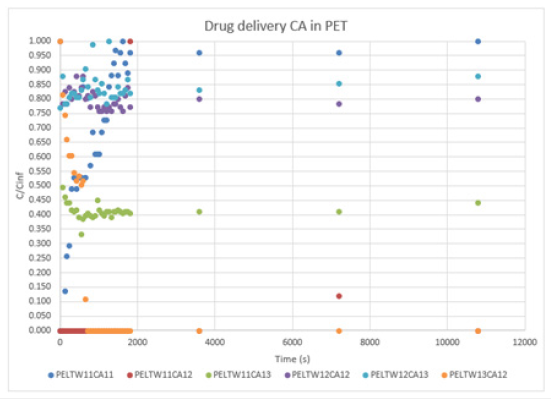
a. PET: The results of Graph 2 show different behave. The changes in ratios of surfactants and crosslinkers affect both aspects. The morphology of the microcapsules and the drug delivery. In this case, the fabric PET reflects very interesting aspects. At first glance, we can see that is not all the drug delivers represented in this Graph 2. Some samples as PELTW12CA11, PELTW13CA11, and PELTW13CA13 reported null values. In this graph, we can see three types of behaving: Type (I): the curve starts with a positive slope until reaches a maximum (Higuchi diffusion), which, will stay for a long period (reservoir effect). Samples as PELTW11CA11, PELTW12CA13, and PELTW13CA12 follow this behave. Type (II), the curve makes a peak of concentration and drop, sample PELTW11CA12 have this type of behaving. The curve has a big peak of concentration delivered and is followed by a drop of this concentration. Type (III), the curves describe by PELTW11CA13 and PELTW13CA12, shows at the first value his maximum concentration and drops. We can extract that the type of drug delivery depends on the ratio between surfactant, crosslinker, and EO. In this fabric, the results show a very tendency to increase his concentration along and stay at this concentration. Type I is only described when the crosslinker is capable of around the microcapsules. If the crosslinker is not capable to do this, the microcapsules tend to release his EO very quickly (type II and III), to retain his EO due to his thick width (Null cases) or the impregnation has low efficiency.
Graph 2:Drug delivery CA in PET.
b. COT: The results of COT tend to have more type III, as we can see in Graph 3. When the ratio of B-CD and crosslinker is 1:3, the results are null. Can happen if the EO cannot be released due to his thick wall. The fabrics tend to have more stable behaviors and more concentration. The sample COLTW11CA11 has type II and III, the microcapsules have a huge peak, drops, peak, and finally drops at “zero”. Two types of microcapsules exist, ones that release the EO very quickly and others that need more energy to break the wall. COLTW13CA13 has just a peak and a drop. The tiny microcapsules are the only ones that could react. Meanwhile, the big ones need more energy and time to release the EO.
Graph 3:Drug delivery CA in COT.

c. PA: Graph 4, shows much lower concentration levels. In comparison with COT. But the curves follow type III. The series LTW12CA seems to be the best. The other cases have a null effect on the media. The PA fabric releases the EO very quickly with the same pattern.thick width (Null cases) or the impregnation has low efficiency.
Graph 4:Drug delivery CA in PA.

Microcapsules of TA
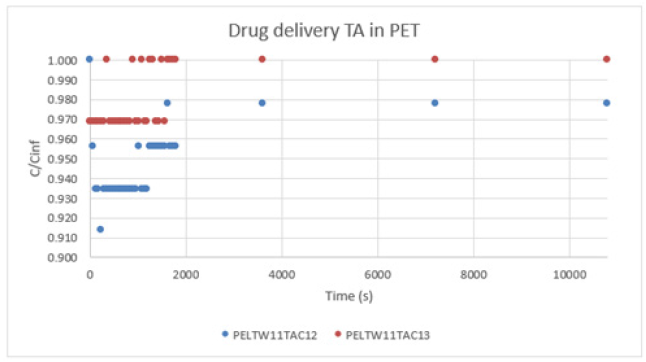
a. PET:Graph 5, shows a type I. The concentration levels are very high. As we know, the TA has very high efficiency of impregnation. But the TA can agglomerate and create a very thick wall.
Graph 5:Drug delivery TA in PET.
b. COT: Graph 6, shows a type I. The results are very similar to the PET case. The concentration levels still are very high. But the sample curve of COLTW11TA11 seems to have a retard on the release, using the same pattern..
Graph 6:Drug delivery TA in COT.
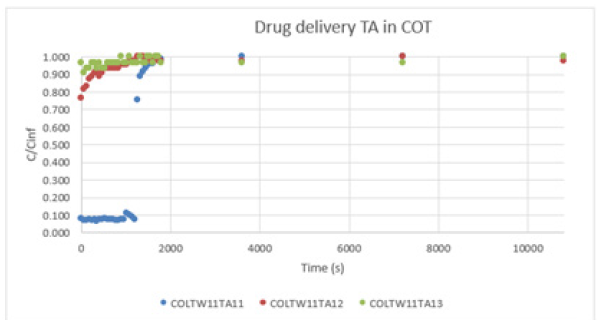
Graph 7, shows a type I. The results continue the pattern as in PET and COT fabric cases. The concentration levels are still very high. Table 2 confirms that all the drug deliveries follow a quasi Fickian model. This means that the order is lower than “0,5”. Overall, the PET and COT fabric is more likely to have a Fickian model. On the contrary, PA shows a low order value. CA and TA microcapsules have differences in drug delivery. CA microcapsules are very adaptable to all the fabrics. On the other hand, TA shows more adaptability on the fabric in the case of PET. But COT and PA show very low values (Table 3).
Graph 7:Drug delivery TA in PA.
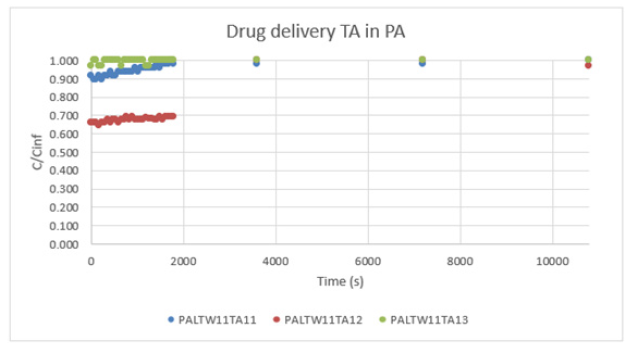
Table 2: Particle size.
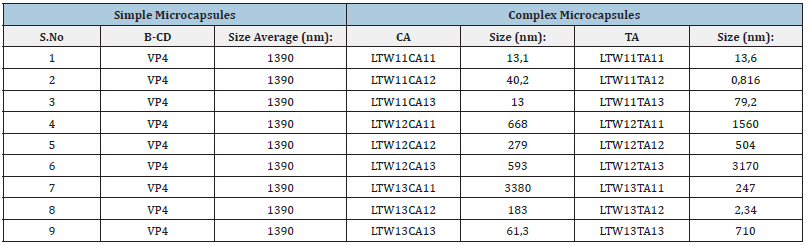
Table 3: Treated results from drug delivery using Korsmeyer-Peppas.

Conclusion
In conclusion, the results of morphology and drug delivery show that it is possible to modify his chemical and physical properties. The simple microcapsules of B-CD present non-industrial applications. This microcapsule shows no homogeneity in size and stability. On the contrary, the use of surfactant and crosslinkers improve his homogeneity on size particle and drug delivery. The PET and COT show a relatively acceptable order of delivery in both cases (CA and TA). PA shows low concentration levels in the case of CA and a very low order value in all cases. The microcapsules have a potential industrial application. But the research must continue. The ratios of EO, surfactant and more crosslike must be more accurate. Taking into account his CMC (critical micelle concentration). And try to use both crosslinkers.
References
- Bombe K (2021) Microencapsulation Market to Reach $17.31 billion by 2027- Exclusive Report Covering Pre and Post COVID-19 Market Analysis by Meticulous Research.
- Kalemba D, Kunicka A (2005) Antibacterial and antifungal properties of essential oils. Current Medicinal Chemistry 10(10):8 13-829.
- Burt S (2004) Essential oils: Their antibacterial properties and potential applications in foods - A review. International Journal of Food Microbiology 94(3): 223-253.
- Bakkali F, Averbeck S, Averbeck D, Idaomar M (2008) Biological effects of essential oils--a review. Food Chem Toxicol 46(2): 449-475.
- Hasheminejad N, Khodaiyan F, Safari M (2019) Improving the antifungical activity of clove esential oil encapsulated by chitosan nanoparticles. Food Chemistry 275: 113-114.
- Oxley J (2014) Microencapsulation in the food industry. In: Sobel R (Ed.), Overview of microencapsulation process technologies. San Antonio, American Print, TX, USA, 10-23.
- Borges Valle JA, Siqueira Curto Valle RD, Krause Bierhalz AC, Maestá Bezerra F, Lopez Hernandez A, et al. (2020) Chitosan microcapsules: Methods of the production and use. Applied Polymer Science 138(21): 10-21.
- Narita T, Yamamoto T, Hosoya E, Dobashi T (2003) Gibbs free energy expression for the system polystyrene in methylcyclohexane and its application to microencapsulation. American Chemical Society 19(13): 5240-5245.
- Islam Shishir MR, Xie L, Sun C, Zheng X, Chen W (2018) Advances in micro and nano-encapsulation of bioactive compounds using biopolymer and lipid-based transporters. Trends in Food Science & Technology 78: 34-60.
- Eghbal N, Choudhary R (2017) Complex coacervation: encapsulation and controlled release of active agents in food systems. LWT - Food Science and Technology, pp. 254-264.
- Rodríguez J, Martín MJ, Ruiz MA, Clares B (2016) Current encapsulation strategies for bioactive oils: From alimentary to pharmaceutical perspectives. Food Research International 83: 41-59.
- Piccinino D, Capecchi E, Botta L, Mattia Bizzarri B, Bolella P, et al. (2018) Layer-by-layer preparation of microcapsules and nanocapsules of mixed polyphenols with high antioxidant and UV-shielding properties. American Chemical Society 19(9): 3883-3893.
- Arshad Hussain S, Dey B, Bhattacharjee D, Mehta N (2018) Unique supramolecular assmebly through Langmuir-Blodgett (LB) technique. Heliyon 4(12): e01038.
- Zheng Y, Zheng M, Ma Z, Xin B, Guo R, et al. (2015) Sugar fatty acid esters. In: Moghis UA, Xu X (Eds.), Polar lipids. Shanghai: Elsevier, China, p. 216.
- Manjanna K, Shivakumar B, Pramod Kumar TM (2010) Microencapsulation: An acclaimed novel drug-delivery system for NSAIDs in arthritis. Begell House 27: 509-545.
- Zhang T, Luo Y, Wang M, Chen F, Liu J, et al. (2020) Double-layered microcapsules significantly improve the long-term effectiveness of essential oil. Polymers 12(8): 1651.
- Anaya-Castro MA, Ayala-Zavala JF, Muñoz-Castellanos L, Hernández-Ochoa L, Peydecastaing J, et al. (2017) β-Cyclodextrin inclusion complexes containing clove (Eugenia caryophyllata) and Mexican oregano (Lippia berlandieri) essential oils: Preparation, physicochemical and antimicrobial characterization. Food Packaging and Shelf Life 14: 97-101.
- Ge Y, Ge M (2016) Distribution of Melaleuca alternifolia essential oil in liposomes with Tween 80 addition and enhancement of in vitro antimicrobial effect. Journal of Experimental Nanoscience 5(11): 345-358.
- Beşen BS (2019) Production of disposable antibacterial textiles via application of tea tree oil encapsulated into different wall materials. Fibers and Polymers 20: 2588-2592.
- Siepmann J, Kranz H, Bodmeier R, Peppas NA (1999) HPMC-matrices for controlled drug delivery- A new model combining. pp. 1748-1756.
- Lamprecht A, Hiromitsu Y, Hirofumi T, Yoshiaki K (2003) Microsphere design for the colonic delivery of 5-fluorouracil. Journal of Controlled Release 90(3): 313-322.
- Siepmann J, Peppas NA (2001) Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv Drug Deliv Rev 48(2-3): 139-157.
- Andreetta Héctor A (2003) Long-acting drugs: release mechanisms. Uses of different models. Buenos Aires Pharmaceutical Act 22(4): 355-363.
- Siepmann J, Siepmann F (2012) Modeling of diffusion-controlled drug delivery. Journal of Controlled Release 161(2): 351-362.
- Siepmann J, Kranz H, Bodmeier R, Peppas NA (1999) HPMC-matrices for controlled drug delivery- a new model combining diffusion, swelling, and dissolution mechanisms and predicting the release kinetics. Pharm Res 16(11): 1748-1756.
© 2022 Lis Arias MJ. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






