- Submissions

Full Text
Polymer Science: Peer Review Journal
Nanocellulose-Based Materials in the Removal of Contaminants from Water
Belkis Sulbarán-Rangel*
Department of Water and Energy, Campus Tonalá, University of Guadalajara, México
*Corresponding author: Belkis Sulbarán- Rangel, Department of Water and Energy, Campus Tonalá, University of Guadalajara, México
Submission: June 20, 2022;Published: July 12, 2022

ISSN: 2770-6613 Volume3 Issue5
Abstract
Water is an essential element for the existence of life and therefore it is an important issue. Due to the diversity of contaminants that can occur in drinking water, water treatment systems are very diverse; within these is the use of adsorbent and filter materials. The objective of this review is to give an overview of the use of nanocellulose-based materials in the removal of contaminants present in water. In general, it has been seen that for a better performance of nanocellulose-based materials for applications in membranes or as an adsorbent, good compatibility between the nanocellulose and the matrix with other polymers is necessary. In addition, the interconnection and formation of networks between fibers increase the porosity and consequently the flow of water. However, it is a significant challenge to maintain the structural integrity of the membrane without compromising water flow and contaminant removal efficiency. Modified nanocellulose derivatives allow the formation of active sites that show a higher affinity towards contaminant molecules through van der Waal forces, electrostatic interaction and hydrogen bonds. These active sites play a vital role in the adsorption of various contaminants, including heavy metal ions, toxic dyes, oils, unwanted salts, etc.
Keywords: Biomaterials; Adsorption; Filtration; Chemical modification; Water contaminants
Introduction
Cellulose is the main component of lignocellulosic biomass, and it is a linear biopolymer formed by 100 to 14,000 glucose subunits linked by β-1,4 glycosidic bonds; the β configuration allows to form very long and resistant chains [1]. Between 1011 and 1012 tons of cellulose are produced annually, mainly from sources of plant origin. However, other organisms from different kingdoms are also capable of synthesizing it [2]. Cellulose can be modified on a nanometric scale by different methods and nanocellulose is obtained and depending on the source of production or method of formation, nanocellulose can be of three types: Cellulose Nanofibrils (CNF) [3], Cellulose Nanocrystals (CNC) [4,5], and Bacterial Nanocellulose (BNC) [6]. CNF and CNC are produced when mechanical and chemical processes are used respectively in the cellulose pulp and are defined as the set of individual nanoparticles with one dimension in the nanometer [7]. The BNC is an extracellular polymer synthesized by bacteria mainly of the genus Acetobacter [8,9].
It is well known that cellulose fibers have had a long tradition as a filter material [10]. Indeed, many of the operations that are carried out daily in a laboratory depend on the filtration processes that are carried out with cellulose-based filter papers. However, there are limitations when it comes to the removal of small molecular weight compounds [11]. This is why, in recent years, nanocellulose has gained considerably more attention due to its outstanding mechanical and chemical properties [12] coupled with its low cost, natural abundance and biocompatibility [13]. That is why in this mini-review a series of works related to the subject is presented, where the various investigations where nanocellulose-based materials have been used for the removal of contaminants in the water are analyzed.
Nanocellulose in Contaminant Removal
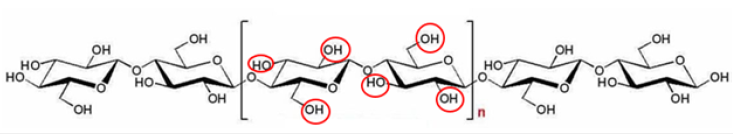
Conventional water treatment techniques such as sedimentation, flocculation, coagulation, and activated carbon cannot effectively remove all contaminants present in water. Therefore, the need for a continuous search for new advanced materials for water treatment continues [14]. Nanocellulose is an excellent and sustainable material that can be surface modified to remove specific contaminants from water due to the presence of hydroxyl groups in its structure (Figure 1) [15]. It is possible to remove various contaminants in the water, such as bacteria, viruses, dyes, heavy metals, etc., through electrostatic attraction through the incorporation of various chemical functionalities [16]. Recent research has shown that nanocellulose can be an alternative in water purification systems. Several recent publications have reported the use of CNC modified with 2,2,6,6-tetramethylpireridine-1-oxyl (TEMPO) and amination, mixed with composite polymers such as Polyethersulfone (PES) and polyamide. In the case of CNC and polyamide-made membrane, these became rougher and more hydrophilic, which would lead to a higher yield compared to the control without CNC. By increasing the amount of CNC in the membranes, the water flux increased significantly by about 35% and the removal of salts such as Na2SO4 remained above 98% and that of MgSO4 above 96%, demonstrating good compatibility between the CNC and the polyamide matrix [17]. Jonoobi et al. prepared and characterized CNC-based composite membranes functionalized with amines and Polyethersulfone (PES) and determined a reduction in chemical oxygen demand and removal of color from wastewater, a by-product of the liquor industry.
Figure 1:Chemical structure of cellulose. The red circles indicate the hydroxyl groups [15].
Bacterial nanocellulose has also been used to make water remediation systems to adsorb heavy metals, oils, and dyes. The BNC and Polydopamine (PDA) are fabricate scalable and reusable films capable of adsorbing toxic dyes such as rhodamine 6G, methyl orange, methylene blue, and heavy metal ions [18]. Other studies have reported wood-derived nanopaper and bacterial cellulose in an ultrafiltration system for water purification, concluding that the size of the pores and the permeate in an ultrafiltration membrane can be controlled by using different types of nanocellulose fibrils [11]. Another interesting study is the one reported the fabricated of crosslinked membranes forming a three-dimensional network composed of BNC and poly 2-methacryloyloxyethylphosphorylcholine (P-MPC). P-MPC is a non-toxic polymer due to its methacrylic functional group and its zwitterionic phosphorylcholine moiety, which consisted of a trimethylammonium cation and a phosphate anion, makes membranes develop bioinert, antimicrobial and antifouling properties [19].
Membranes Composed of Nanocellulose and Other Polymers
In the same way, microfiltration membranes based on cellulose nanofibers composed of polyacrylonitrile and electrospun polyethylene terephthalate have been developed, for the elimination of bacteria, viruses and heavy metals. After filtration, the authors showed complete removal of Escherichia coli bacteria, these membranes also exhibited an adsorption capacity of 100mg chromium and 260mg lead and a log reduction value of 4 for MS2 virus removal [20]. Other TEMPO oxidation cellulose nanofiber membranes were developed to perform MS2 bacteriophage tests and demonstrated that TEMPO NFCs have a high virus adsorption capacity, which they attributed to the surface volume ratio and the negatively charged surface of NFCs [21]. Other authors reported on the use of jute cellulose nanofiber membranes compounded with polyacrylonitrile for wastewater purification; filtered silica nanoparticles and oil/water separation, concluding that after filtration, the membranes successfully removed silica nanoparticles from 7 to 40nm. In addition, they showed that composite NFC membranes exhibited 99.5% rejection against the oil/water mixture. In addition, they observed that the concentration of oil in the filter was less than 5.5ppm, this value complied with the environmental standards for the discharge of residual water, which is reported to be (<10ppm) [22].
Likewise, agave bagasse cellulose nanofiber membranes prepared with TEMPO oxidation have been studied, in a water purification process, for the elimination of an emerging contaminant (ciprofloxacin) and they verified that the nanofibers could remove the 27.7% of the contaminant [23]. Nanocellulose has also been used to remove dyes and toxic contaminants from water. Membranes prepared with a double layer of Graphene Oxide (GO) and one of CNF without using any chemical crosslinkers. In this investigation, they observed that with the incorporation of a layer of GO sheets on the CNF membrane and through the sequential filtration of water, the performance of the membrane improved. These membranes also showed dye filtration greater than 90%. It had also been observed that the use of a thin layer of GO as a functional layer in CNF could provide synergistic membranes mainly due to the strong interactions between the two, due to the formation of inclusive networks of inter- and intra-molecular hydrogen bonds [24]. The manufacture and use of a membrane from carbonized cellulose nanofibers have been reported, showing that carbonized CNF membranes were able to remove methylene blue efficiently [25]. In our research, the preparation of electrospun organic membranes from CNF obtained from agave bagasse fibers and Polycaprolactone (PCL) has been reported. In this investigation, it was reported that the adsorption of heavy metals was due to the negative surface charge of the membranes, and the turbidity and conductivity of water for human consumption were also reduced. Electrospun PCL50:NFC50 membranes not only removed 100% of conductivity and turbidity but also successfully removed a good percentage of heavy metals (Cr=99% and Fe=75%) that were present in tap water [26].
Conclusion
The applications of nanocellulose as a base for materials used in water purification and remediation are considered to be advancing every day since it has been shown that these nanomaterials can be used in current technologies and even complement conventional ones. The research highlighted in this review suggests that nanocellulose shows excellent compatibility with other polymers and the processing requirements for preparing nanocellulosebased composites are minimal. Since water quality is a global concern, the use of natural and sustainable materials is essential to obtain high-value products that can remediate water systems without generating other sources of pollution or requiring extra energy inputs or high secondary costs. Filtration systems can often provide an ideal alternative to conventional water treatment. The inherent variety of functional groups possessed by nanocellulose provides better interaction with certain types of contaminants. Thus, nanocellulose-based materials have been used to generate filtration and adsorption systems, among other options in water treatment.
References
- Dufresne A, Dupeyre D, Vignon MR (2000) Cellulose microfibrils from potato tuber cells: Processing and characterization of starch–cellulose microfibril composites. Journal of Applied Polymer Science 76(14): 2080-2092.
- Chávez-Pacheco JL, Martínez Yee S, Contreras ZM, Escamilla ME (2004) Bacterial cellulose in gluconacetobacter xylinum: biosynthesis and applications. Tip Specialized Journal in Chemical-Biological Sciences 7(1): 18-25.
- Palacios HH, Hernandez DJA, Esquivel AM, Toriz G, Rojas OJ, et al. (2019) Isolation and characterization of nanofibrillar cellulose from agave tequilana weber bagasse. Advances in Materials Science and Engineering 7.
- Hernández J, Romero VH, Escalante A, Toriz G, Rojas O, et al. (2018) Agave tequilana bagasse as source of cellulose nanocrystals via organosolv treatment. BioResources 13(2): 3603-3614.
- Rebouillat S, Pla F (2013) State of the art manufacturing and engineering of nanocellulose: A review of available data and industrial application. Journal of Biomaterials and Nanobiotechnology 4: 165-188.
- Sharma C, Bhardwaj NK (2019) Bacterial nanocellulose: Present status, biomedical applications and future perspectives. Materials Science and Engineering: C 104: 109963.
- Hubbe M, Rojas O, Lucia L, Sain M (2008) Cellulosic nanocomposites: A review. BioResources 3(3).
- Castro C, Zuluaga R, Álvarez C, Putaux JL, Caro G, et al. (2012) Bacterial cellulose produced by a new acid-resistant strain of Gluconacetobacter genus. Carbohydrate Polymers 89(4): 1033-1037.
- Lee KY, Aitomäki Y, Berglund LA, Oksman K, Bismarck A (2014) On the use of nanocellulose as reinforcement in polymer matrix composites. Composites Science and Technology 105: 15-27.
- Baker M (1949) The quest for pure water: the history of water purification from the earliest records to the twentieth century American Water Works Association: Washington, USA.
- Mautner A, Lee KY, Tammelin T, Mathew AP, Nedoma AJ, et al. (2015) Cellulose nanopapers as tight aqueous ultra-filtration membranes. Reactive and Functional Polymers 86: 209-214.
- Klemm D, Heublein B, Fink HP, Bohn A (2005) Cellulose: fascinating biopolymer and sustainable raw material. Angewandte chemie international edition 44(22): 3358-3393.
- Gopakumar DA, Thomas S, Grohens Y (2016) Chapter 8 - Nanocelluloses as innovative polymers for membrane applications. In: Puglia D, Fortunati E, Kenny JM (Eds.), Multifunctional polymeric nanocomposites based on cellulosic reinforcements. William Andrew Publishing, USA, pp. 253-275.
- Bonadies I (2019) Chapter 8 - Nanoscale materials in water purification. In: Thomas S, Pasquini D, Leu SY, Gopakumar DA (Eds.), Nanoscale materials in water purification. Elsevier, Netherlands, pp. 231-246.
- Siqueira G, Bras J, Dufresne A (2009) Cellulose whiskers versus microfibrils: influence of the nature of the nanoparticle and its surface functionalization on the thermal and mechanical properties of nanocomposites. Biomacromolecules 10(2): 425-432.
- Gopakumar DA, Arumughan V, Pasquini D, Leu SY, Thomas S (2019) Chapter 3 - Nanocellulose-based membranes for water purification. In: Thomas S, Pasquini D, Leu SY, Gopakumar DA (Eds.), Nanoscale materials in water purification. Elsevier, Netherlands, pp. 59-85.
- Huang S, Wu MB, Zhu CY, Ma MQ, Yang J, et al. (2019) Polyamide nanofiltration membranes incorporated with cellulose nanocrystals for enhanced water flux and chlorine resistance. ACS Sustainable Chemistry & Engineering 7(14): 12315-12322.
- Gholami DH, Jiang Q, Ghim D, Cao S, Chandar YJ, et al. (2019) A robust and scalable polydopamine/bacterial nanocellulose hybrid membrane for efficient wastewater treatment. ACS Applied Nano Materials 2(2): 1092-1101.
- Vilela C, Moreirinha C, Almeida A, Silvestre AJD, Freire CSR (2019) Zwitterionic nanocellulose-based membranes for organic dye removal. Materials 12(9): 1404.
- Wang R, Guan S, Sato A, Wang X, Wang Z, et al. (2013) Nanofibrous microfiltration membranes capable of removing bacteria, viruses and heavy metal ions. Journal of Membrane Science 446: 376-382.
- Ma H, Burger C, Hsiao BS, Chu B (2011) Ultrafine polysaccharide nanofibrous membranes for water purification. Biomacromolecules 12(4): 970-976.
- Cao X, Huang M, Ding B, Yu J, Sun G (2013) Robust polyacrylonitrile nanofibrous membrane reinforced with jute cellulose nanowhiskers for water purification. Desalination 316: 120-126.
- Sulbarán RBC, Madrigal Olveira AE, Romero Arellano VH, Guzmán González CA (2019) Cellulosic nanomaterials for the adsorption of emerging contaminants. Technure 23(62): 13-20.
- Liu P, Zhu C, Mathew AP (2019) Mechanically robust high flux graphene oxide - nanocellulose membranes for dye removal from water. Journal of Hazardous Materials 371: 484-493.
- Liang HW, Cao X, Zhang WJ, Lin HT, Zhou F, et al. (2011) Robust and highly efficient free-standing carbonaceous nanofiber membranes for water purification. Advanced Functional Materials 21(20): 3851-3858.
- Palacios H, Urena-SH, Zurita F, Guerrero de León AA, Sundaram G, et al. (2020) Nanocellulose and polycaprolactone nanospun composite membranes and their potential for the removal of pollutants from water. Molecules 25(3): 683.
© 2022 Belkis Sulbarán-Rangel. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






