- Submissions

Full Text
Polymer Science: Peer Review Journal
Chemical Mesoscopics for the Reduction– Oxidation Reactions Results Explanation
Kodolov VI1,2* and Kodolova-Chukhontzeva VV1,3
1Basic Research - High Educational Centre of Chemical Physic & Mesoscopics, Russia
2M T Kalashnikov Izhevsk State Technical University, Russia
3Peter Great St Petersburg Polytechnic University, Russia
*Corresponding author: Kodolov VI, Basic Research - High Educational Centre of Chemical Physic & Mesoscopics and M T Kalashnikov Izhevsk State Technical University, Izhevsk, Russia
Submission: March 01, 2021;Published: June 09, 2022

ISSN: 2770-6613 Volume3 Issue5
Abstract
The new scientific trend, Chemical Mesoscopics, is based on the ideas of the Mesoscopics (Mesoscopic Physics) and also on the Mesoscopic Chemistry. This trend is considered on the instances on the reductionoxidation reactions peculiarities with nanostructures and mesoparticles participation. In this case such notions as the interference (the chemical bond formation), and the annihilation which stimulates the electron shift and leads to the chemical bond’s formation, and also atom magnetic moment growth, are discussed. In this paper the explanation of the examples of the mesoscopic particles characteristics changes because of the Red Ox processes are given.
Keywords:Chemical reactions; Charge quantization; Interference; Annihilation; Nanostructures reactivity; Red ox reactions; Atomic magnetic moments; Kolmogorov-avrami equations Changed
Introduction
Chemical Mesoscopics as new trend in Chemical Sciences was appeared from such scientific trends as Synergetics (Self Organization), Fractal Theory (Self Similarity), Theories of Chemical Kinetics and Catalysis [1-3]. All of these trends are used for the description of mesoscopic particles (or nanostructures) behavior in the different media and at the various condition’s changes. Therefore, the Mesoscopic Physics and later appeared Mesoscopic Chemistry can be presented as the basis of Chemical Mesoscopics. Above new trend is very near to Chemical Physics on the considered objects and also on the phenomena and particularities of the various reactions and processes at the changes of conditions of them realization. However, the basic aim of this advanced trend is appeared the investigation of nanostructures (or mesoparticles) reactivity in the various media and at the different changed conditions.
About the Annihilation Phenomenon at Red–Ox Reactions and at the Modification of Polymeric Compositions
At the modification of the nanostructures and polymeric materials the process of annihilation creation is occurred by the following actions: the negative charge quants are directed to positive charged atom, near nucleus which the positive charge quants are located, and interact with them. As a result, the annihilation with the electromagnetic direct field formation takes place. This field stimulates the electron shift with the activation of negative charge quants and then the interference of these quants (electron waves) with the new chemical bond’s formation. Thus, the reduction oxidation processes can be explained by two phenomena: annihilation and interference following one after another. In this case it’s possible the d electron despairing with the spin formation accompanied by the magnetic moment growth. The above ideas are considered on the examples of the metal carbon mesoscopic composites modification reactions and also different polymeric materials. The hypothesis about possibility of annihilation at the interaction of positive and negative charges quants in red ox processes is confirmed by the examples of processes of Copper and Nickel Carbon mesocomposites modification with application such substances as polyethylene polyamine, ammonium iodide, Ammonium Polyphosphate (APP), silica (SiO2), aluminium oxide, iron oxide, nickel oxide and copper oxide [7-9]. In the case, when polyethylene polyamine and ammonium iodide are applied, the connection reactions take place. At the interactions of polyethylene polyamine with mesoparticles the C=N bond formation is explained by the interference of negative charges quants. When the mesoparticles modification reactions with the using APP, SiO2, metal oxides are carried out, the redox processes are realized. In these cases, the modifiers reduction reactions take place. The structures of metal carbon mesoscopic composites with active carbon shells are defined by means of the complex of methods including x-ray photoelectron spectroscopy, transition electron microscopy with high permission, electron microdiffraction and also EPR spectroscopy [4-11].
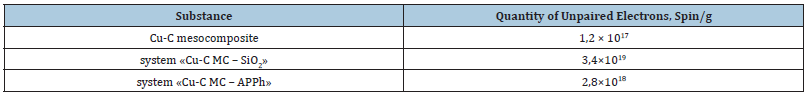
Below in Tables 1 & 2 the examples of metal atomic magnetic moments changes (in Boron magnetons) and quantities of unpaired electrons (in spin/g) for mesoparticles modified by APPh or silica after the mechanochemical modification processes proceeding are given. The metal atomic magnetic moment growth proceeds owing to the redox processes with above chemical compounds. The assignment of active nanostructures (mesoparticles) during the composition’s modification is concluded in the activation of matrices self-organization in needful direction. For the realization of this goal the determination of organized phase part is necessary. In some papers [12-20] the positive results on materials properties improvement are presented when the minute quantities of metal carbon mesocomposites are introduced in these materials. In paper [17] the hypothesis about nanostructures influence transmission on macromolecules of polymeric matrices is proposed. This hypothesis is complied with mesoscopic physics principles which consider quantum effects at the certain conditions of mesoparticle existence. The composition polarization is possible because of there is the charge quantization with the wave expansion on polar (functional) groups of media (for example, polymer macromolecule). The quantum charge wave expansion leads to the functional groups’ polarization (dipole moments) change as well as the extinction increasing [21,22].
Table 1:The values of Copper (Nickel) atomic magnetic moments in the interaction products for systems: Cu-C MC – APPh (or SiO2) and Ni-C MC – APPh (or SiO2).
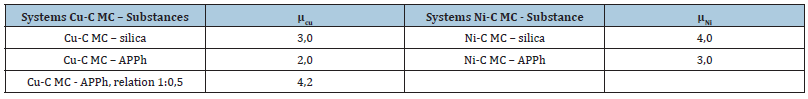
Table 2:he unpaired electron values (from EPR spectra) for systems “Cu-C MC – silica” and “Cu-C MC – APPh” (relation 1:1) in comparison with initial mesoparticle Cu-C MC.
Conclusion
On the base of investigation results discussion, it may conclude that the new scientific trend, Chemical Mesoscopics, considers the nanostructures (or mesoparticles) reactivity, their formation and also the processes with these particles’ participation. The development this trend supposes the theory development for mechanisms of mesoscopic systems formation as well as mechanisms of self-organization in media and compositions. The peculiarities of structures and energetic characteristics of mesocomposites obtained cause their possibilities for applications in the different fields. The examples of following applications in radical, red ox and addition processes as catalysts, reagents and also inhibitors as well as additives and modifiers improving properties of materials (inorganic and organic polymeric materials), adhesives and glues, fireproof systems, corrosion inhibitors, medicine magnetic transport remedies, stimulators of plant growth are presented in [23]. The Metal Carbon mesoscopic composites owing to their magnetic characteristics can be used in the electromagnetic radiation focal systems. This unique scientific trend discovers new era in the development of new theories in the natural sciences and in the practice, for instance, novel nanostructures application widening.
References
- Kodolov VI, Trineeva VV (2017) New scientific trend – Chemical mesoscopics – Chemical Physics & Mesoscopics. 19(3): 454- 465.
- Kodolov VI, Kodolova-CVV, Terebova NS, Shabanova IN (2019) The explanation of magnetic metal carbon mesocomposites synthesis peculiarities by means of mesoscopic notions. Academ J Polym Sci 3(2): 555613.
- Moskaletz MV (2010) Fundamentals of mesoscopic physics – Khar’kov: NTU KhPI, Ukraine, p. 180.
- Ruedenberg K (1964) Physical nature of chemical bond. M: Publ “Mir”, Russia, p. 162.
- Kodolov VI, Kodolova-CVV, Shabanova IN (2020) Review: Possible fields of metal carbon mesoscopic composites application. In: Innovations and challenges in modern physical chemistry: Research and practice. Nova Sci Publ, USA.
- Kodolov VI, Trineeva VV, Pershin YV (2020) Method of metal carbon nanocomposites obtaining from metal oxides and polyvinyl alcohol. Pat RU 2018122 001.
- Kodolov VI, Kodolova-CVV (2019) Fundamentals of chemical mesoscopics – Monograph. - Izhevsk: Publisher – M.T. Kalashnikov Izhevsk State Technical University, Russia, p. 218.
- Mustakimov RV, Kodolov VI, Shabanova IN, Terebova NS (2017) Modification of copper carbon nanocomposites with the using of Ammonium Polyphosphate for the application as modifiers of epoxy resins. Chemical Physics & Mesoscopics 19(1): 50-57.
- Kodolov VI, Trineeva VV, Kopylova AA (2017) Mechanochemical modification of metal carbon nanocomposites. Chemical Physics & Mesoscopics 19(4): 569-580.
- Kodolov VI, Trineeva VV, Terebova NS (2018) The change of electron structure and magnetic characteristics of modified copper carbon nanocomposites. Chemical Physics & Mesoscopics 20(1): 72-79.
- Shabanova IN, Kodolov VI, Terebova NS, Trineeva VV (2012) X ray electro spectroscopy in investigation of metal/carbon nanosystems and nanostructured materials. Izhevsk–Moscow: Publ Udmurt University, Russia, p. 252.
- Shabanova IN, Terebova NS, Kodolov VI (2013) The investigation of metal or carbon nanocomposites electron structure by X ray photoelectron spectroscopy. In: Nanostructure, nanosystems and nanostructured materials. Theory, production and development. Apple Academic Press, USA, pp. 177-230.
- Kodolov VI, Lipanov AM, Trineeva VV (2013) The changes of properties of materials modified by metal/carbon nanocomposites. Ibid, pp. 327-373.
- Kodolov VI, Trineeva VV (2013) Theory of modification of polymeric materials by super small quantities of metal/carbon nanocomposites. Chemical Physics & Mesoscopy 15(3): 351-363.
- Kodolov VI, Trineeva VV (2012) Perspectives of idea development about nanosystems self-organization in polymeric matrixes. In: The problems of nanochemistry for the creation of new materials. Torun, Poland, pp. 75-100.
- Akhmetshina LF, Lebedeva GA, Kodolov VI (2012) Phosphorus containing metal/carbon nanocomposites and their application for the modification of intumescent fireproof coatings. Journal of Characterization and Development of Novel Materials 4(4): 451-468.
- Kodolov VI, Kovyazina ОА, Trineeva VV, Vasilchenko YM, Vakhrushina МА, et al. (2010) On the production of metal/carbon nanocomposites, water and organic suspensions on their basis. VII International Scientific-Technical Conference Nanotechnologies to the Production, Proceedings. Fryazino, Russia, pp. 52-53.
- Chashkin MA (2012) Peculiarities of modification by metal/carbon nanocomposites for cold hardened epoxy compositions and the investigation of properties of polymeric compositions obtained. Thesis of cand diss. Perm: PNSPU, India, p. 17.
- Kodolov VI, Kodolova VV, Semakina NV, Yakovlev GI, Volkova EG, et al. (2008) Patent 2337062 Russia technique of obtaining carbon nanostructures from organic compounds and metal containing substances.
- Kodolov VI, Trineeva VV, Kovyazina OA, Vasilchenko YM (2012) Production and application of metal. Carbon Nanocomposites. In: The problems of nanochemistry for the creation of new materials – Torun, Poland, pp. 23-36.
- Akhmetshina LF, Kodolov VI, Tereshkin IP, Korotin AI (2010) The influence of carbon metal containing nanostructures on strength properties of concrete composites. Internet Journal Nanotechnologies in Construction 6: 35-46.
- Pershin YV, Kodolov VI (2012) Polycarbonate modified with Cu-C nanocomposite. In: The problems of nanochemistry for the creation of new materials. Torun: Publ. IEPMD, Poland, pp. 173-179.
- Kodolov VI, Kodolova-CVV, Shabanova IN (2020) Review: Possible Fields of metal carbon mesocomposites application. – In Book: Innovation and Challenges in Modern Physical Chemistry. Research and Practice. – N.Y.: Nova Sciences Publishers, Inc., 2020, 195p. – Pp. 57–122.
© 2022 Kodolov VI. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






