- Submissions

Full Text
Polymer Science: Peer Review Journal
Bio-Based Polyamides: A Commercial Demonstration of Synthetic Biology
Alexander Kedo and Xiucai Liu*
Cathay Biotech Inc, Shanghai, China
*Corresponding author: Xiucai Liu, Cathay Biotech Inc, No.1690, Cailun Rd, Pudong District, Shanghai, China
Submission: April 29, 2022;Published: May 27, 2022

ISSN: 2770-6613 Volume3 Issue4
Abstract
Synthetic biology offers the promise of harnessing the power of enzymatic systems to produce new materials, which previously have not been commercially feasible, or existing materials with an improved environmental impact. Improving the environmental impact, as measured by life-cycle analysis, has motivated policy leaders to support bio-manufacturing and has resulted in several commercial projects to test this new technology. Among these projects have been Poly-Butylene Succinate (PBS), Poly-Hydroxyalkanoates (PHA), Polylactic Acid (PLA) ethylene glycol, furan dicarboxylic acid, Long Chain Dicarboxylic Acid (LCDA) and Pentamethylenediamine (PDA). Polycondensation of these latter two monomers produce a new class of polyamides which are renewable, demonstrate a favorable improvement in environmental impact and provide unique processing and performance attributes.
Keywords: Materials; Synthetic biology; Polyamide; Monomer; Polymerization; Fibers
Abbreviations: PDA: Pentamethylenediamine; LCDA: Long Chain Dicarboxylic Acid; PBS: Poly-Butylene Succinate; ZFNs: Zinc-Finger Nucleases; PHA: Poly-Hydroxyalkanoates; PLA: Polylactic Acid
Introduction
Synthetic biology is the combination of biology and other scientific disciplines that leverages the tremendous synthetic capability of naturally occurring biological systems with the goal of commercially producing new materials, heretofore impossible, or existing materials with a considerably improved environmental impact. Synthetic biology, a concept first introduced in the 1980s, became an academic and commercial option in the past two decades due to the development and optimization of basic genetic manipulation techniques, such as Zinc-Finger Nucleases (ZFNs), Transcription Activator-like Effector Nucleases (TALEN), and Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) [1,2]. Increased environmental awareness, concerns of production worker safety and increased costs of raw materials, such as crude oil, have contributed to the growth in policy interest and investment in synthetic biology [3], especially in China, US and Europe. This policy and investment support has positively contributed to the innovation, development, and adoption of bio-produced materials in the market. Selectively modifying naturally occurring biological systems provides the opportunity for the commercially viable production of new molecules previously impossible by traditional chemistry.
Bio-Manufacturing
Bio-manufacturing, or producing materials using bio-processes, has significant advantages
compared to traditional chemistry. Among these include
I) Bioprocesses typically operate at ambient temperatures, pressures and neutral pHs
and therefore reduce the production worker safety risk profile and depreciation costs
compared with chemical manufacturing processes.
II) The catalytic capabilities of enzymes in the bioprocess
far exceed that of traditional catalytic chemistry which allows
production of new chemicals, previously commercially
impossible by traditional chemistry.
III) Bioprocesses are highly specific, which reduces
production of unwanted impurities.
IV) Feedstocks for bioprocesses are often renewable. All
of these contribute to the lower environmental footprint for
bio-manufacturing and explain the revolution in transitioning
chemical manufacturing to bio-manufacturing. Production
cost is a key metric for the commercial viability for biomanufacturing,
and how this cost compares with traditional
petrochemical-based production. A highly efficient bioprocess
is highlighted by several factors, including the conversion
rate, energy consumption, cycle time, separation costs, and
purification costs, all of which must be optimized prior to
commercialization to be successful.
Starting in the 1990s, several business pioneers accepted the bio-manufacturing challenge. Unfortunately, many of these early ventures failed, which include Metabolix’s PHA project, Verdezyne’s dodecanedioic acid project and BioAmber’s succinic acid project. Other bioprocess projects are underway, but are not yet commercial, which include adipic acid [4], and 2,5-Furandicarboxylic acid [5-10], bio-based fuel [11], etc. However, in recent years, both Zymergen and Ginkgo Bioworks successfully integrated robotics and artificial intelligence into the development of synthetic biology and built a new commercial model. Cathay Biotech is among the very few to successfully commercialize synthetic biology.
Bio-Based Polyamide
Inconsistent crude oil supply combined with the negative environmental impact of these resources has motivated the market to consider alternatives to petrol-based products. Among those alternatives are polymers that are produced from renewable raw materials or produced using a process which has a more favorable environmental footprint, including the global warming potential (GWP, kg CO2/kg product). The most common monomer types found in common metabolic pathways are amino acids, alcohols, amines, and acids. From these fundamental monomer categories, bio-based polyesters and polyamides are feasible. Many products that could be used for polyesters have already been well established and include Poly-Butylene Succinate (PBS), Poly-Hydroxyalkanoates (PHA) and Polylactic Acid (PLA) [12-16]. Other biobased monomers such as ethylene glycol [17-20] and furan dicarboxylic acid [5-10] have also been reported. Many of these polyesters demonstrate facile hydrolysis and poor temperature tolerance which limit their applicability. Finally, as petrol-based polyesters are very cost competitive, bio-based polyesters have difficulty competing due to their relatively high production and purification costs.
Polyamides mimic naturally occurring polymers that use the amide linkage, –NH-C=O-, which is a common and important functional group that contributes to the unique properties of peptides, proteins, beta-lactam antibiotics, among others. Considering this, the market has been intrigued by the potential to produce bio-based and renewable polyamides with new unique functionality. Polyamides (PA) are a singular type of polymeric materials that show tremendous mechanical properties and high thermal and chemical stability. Common aliphatic polyamides are normally synthesized by polycondensation reactions of dicarboxylic acids with diamines, or by polycondensation of amino acids or via Ring-Opening Polymerization (ROP) of cyclic amides. Due to the significant and growing application market coupled with the demand for environmentally friendly products, Cathay Biotech built a complete industrial supply chain of bio-based polyamides, from monomer to polymer, from the genome to applied material science. This required establishing a bio-industrial chain from raw material processing through product formulation and solving the many production cost challenges, with the goal of being of similar scale as the petroleum-based chemical industry. After over two decades, leveraging core competencies in genetic engineering, fermentation, chemical and material engineering, Long-Chain Dicarboxylic Acids (LCDA), Penta methylene Diamine (PDA), a family of bio-produced renewable polyamides have been successfully commercialized.
Biotech long-chain dicarboxylic acids
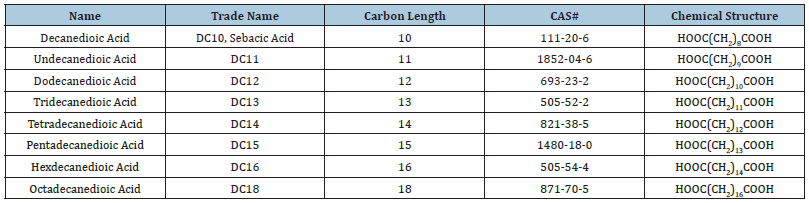
Long-Chain Dicarboxylic Acids (LCDA), aliphatic dicarboxylic acids with over 10 carbons, are one of the most important industrial feedstocks for fine chemistry. Historically, there were three methods for synthesis of LCDAs. The cracking of vegetable oil and chemical synthesis methods provided only a limited carbon length product offering for LCDAs. The microbial fermentation method emerged as the most efficient method with the lowest cost, compared with the other methodologies. Furthermore, fermentation was able to produce LCDAs in the C10-C18 (Table 1), including those carbon lengths previously commercially unattainable by other methods. Cathay produces LCDA at two production plants with a total capacity of 75,000 metric tons annually. The downstream market of LCDA serves a variety of markets globally, including corrosion inhibition, coatings, lubricants, plasticizers, engineering plastics, fragrances, and pharmaceuticals. As an example of the latter, DC16 (the 16 carbon LCDA) and DC18 have application in the production of Semaglutide, a GLP-1 agonist from Novo Nordisk, which is a leading drug for the treatment of diabetes. There are limited substitutes for LCDAs, and growth is anticipated for the foreseeable future.
Table 1:Long-chain dicarboxylic acids currently produced by synthetic biology.
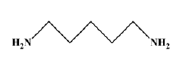
Pentamethylenediamine (PDA)
Pentamethylenediamine (PDA, Figure 1), also known as cadaverine, is naturally generated via the decarboxylation of the amino acid lysine during tissue decomposition. Cathay Biotech has developed proprietary technology to efficiently bio-produce PDA. Currently available feedstocks for chemical synthesis do not allow for the commercially viable production of PDA, which has an odd-numbered five-carbon backbone. As PDA is produced with renewable resources, starting with corn, which is then converted to glucose to provide the carbon source for the downstream fermentation, the resulting carbons in PDA are all renewable and can be quantitatively verified by ASTM 6866. Cathay Biotech has commercialized a PDA production line with a scale of 50,000 metric ton annual capacity. The downstream markets and application for PDA include bio-based polyamides, agricultural chemicals, Penta methylene Diisocyanate (PDI), epoxy hardeners and coatings. The high quality of bio-based PDA has been enthusiastically marketed by a global leader in automotive and consumer coatings with the downstream product receiving the European Coating Symposia Innovation Award, and the British Coating Federation Sustainable Innovation Award.
Figure 1:Pentamethylenediamine.
Long chain diamines (DN10 and DN12)
Decanediamine (DC10) is an important monomer in the production of high-performance polyamides such as PA1012 and PA10T. Dodecanediamine (DN12), with the addition of two carbons, demonstrates less water absorbance and improved processibility for PA12T. Unfortunately, the relative high cost of DN10 and DN12 has limited the application of these very good high-performance monomers. Recent advances enable sebacic acid and dodecanedioic to be converted into DN10 and DN12. This new technology allows for relatively crude grades of each diacid to be used without affecting final diamine product quality and at an attractive market price compared with chemically synthesized DN10 and DN12.
Bio-based polyamide
Polyamides are produced via a condensation reaction of diacid(s) with diamine(s). As Cathay Biotech produces both monomers, this puts Cathay Biotech in a unique position to significantly add to the choices available to today’s polymer chemist and value to the consumer market. These new renewable polyamides cover a wide range of performance attributes as well as offering some new characteristics never seen in high performance polyamides.
Bio-based polyamide 56: Polyamide 6 (PA6) and polyamide 66 (PA66) are the most produce polyamides. Unfortunately, both have limitations, such as high hydrophilicity, low transparency and low tolerance to high temperatures, which limit their application in many areas. However, with the introduction of PDA, a new polyamide is possible when polymerized with adipic acid, Polyamide 56 (PA56, Figure 2). PA56 has a lower hydrogen bond density than PA66, due to the asymmetry of the odd carbon structure of PDA that results in only 50% of the amide groups to generate hydrogen bonds. Furthermore, PA56 has a higher density of amides than PA6 or PA66.
Figure 2: Repeating unit of polyamide 66 and polyamide 56.

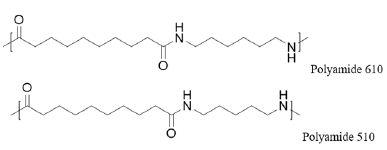
Bio-based polyamide 5X: As mentioned, Cathay Biotech produces a wide range of LCDAs. Polymerization of these with Cathay’s renewable PDA has resulted in a polyamide 5X (PA5X) family which addresses a broad range of market-based functionality while providing a strong environmental-friendly message that is attractive to consumers. For example, with a variety of long carbon chain LCDAs available, specific melting points can be obtained, which optimize production capacities and address specific market niches. Polyamide 510 (PA510, Figure 3) as a 100% bio-based polyamide has been announced by Toray, for application in electronics and textiles. PA510 and polyamide 513 (PA513) performance has compared favorably with polyamide 610 (PA610) and polyamide 612 (PA612) in these applications.
Figure 3: Repeating unit of polyamide 66 and polyamide 56.
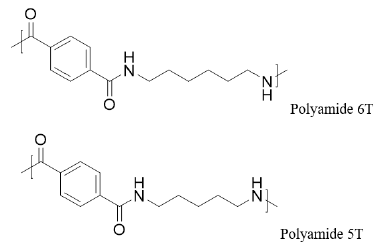
Bio-based polyamide 5T: Semi-aromatic polyamides demonstrate a higher melting temperature and lower water absorbance, making these attractive for engineering polymers. While the raw material cost for polyamide 6T (PA6T) is lower than that for PA66, the market price is the opposite. One explanation for this is the higher polymerization cost for PA6T. One solution offered by PDA (Figure 4) is that this monomer reduces the manufacturing costs by its higher flowability, reducing PA6T from a two-step polymerization into one. By addressing costs, ease of polymerization and processability, these new PDA based terephthalate polyamides provide the market with a high value and cost-effective solution.
Figure 4: Repeating unit of polyamide 6T and polyamide 5T.
Low-cost polyamide 10T and 12T: With the advent of biotechnology and the increased efficiency that this technology brings to the production process for long chain diacids, production costs have decreased. This creates the opportunity to provide polyamide 10T (PA10T) and polyamide 12T (PA12T) at a price point that justifies its application in new products and new markets. Optimization of the cost structure for these new bio-polyamides would require integration of the long chain diacid and diamine production assets. It has been demonstrated that PA12T has less water absorbance and processability that PA10T. Advances in bioprocess technology anticipate PA12T being cost competitive with PA10T
Conclusion
Textile industry: TERRYL®
TERRYL® is a bio-based polyamide launched by Cathay Biotech that is suitable for fibers and the textile industry. The bio-based content has been analytically verified by third-party laboratories using ASTM 6866 methodology to have renewable carbon content in the 45%-100% range depending upon the diacid used in polymerization. The higher renewable content effectively reduces the use of products made from fossil raw materials such as petroleum, and thus reduces carbon emissions. The environmental benefits and the sustainable nature of TERRYL® are not only reflected in its percentage of renewable carbons, but also in the Life Cycle Analysis (LCA) of these products. A portion of the LCA includes the Global Warming Potential (GWP) which is the amount of atmospheric CO2 as kilograms produced per kilogram of the products. The GWP of TERRYL® provides a strong market story valued by environmentally conscious consumers.
TERRYL® fiber products have excellent spinning and finishing properties, due to the unique hydrogen-bond alignment and crystalline state. Compared with polyamide fibers from fossil resources, such as petroleum (e.g., PA6 or PA66), TERRYL® fibers have excellent processability and wearing characteristics such as easy dyeing at low temperatures, soft and skin-friendly feel, good moisture and sweat absorption, good wear and weather resistance. TERRYL®’s natural flame retardant properties also bring unique application advantages. Most importantly, TERRYL® is the only polyamide produced via a melt spinning process, which significantly reduces the production cost.
Engineering plastic: ECOPENT®
PA6 and PA66 are now the most widely used engineering plastics, due to their dense hydrogen bond alignment and crystalline state. But the thermal resistance of PA66 is insufficient for more extreme applications. To address this, PA6T is often a better choice with excellent mechanical properties, and better high temperature tolerance. Unfortunately, PA6T, along with other high-temperature resistant polymers, has limited application because of the high cost and complex producing process. Furthermore, these general weaknesses are shared by every commonly used thermal resistant polymer. PDA based polyamides offer a potential solution to this deficiency, with PA5Xs that have melting temperatures in excess of 300 ℃ combined with other strong applicative properties as well. In addition, due to the unique structural arrangement of PA5X, the producing process is much simpler than PA6T, and at a cost that is comparable with PA66. This PA5X family is well-poised to add significant value to the thermal resistant polyamide market on the basis of their applicative performance, their attractive cost and the very attractive environmentally friendly message available in the product messaging. The structure characteristics of the PA5X family allow for high flowability, which is a desirable characteristic. This enables the bio-based polyamide to be more easily processed and molded. This behavior has application for the thermosetting resin market, such as epoxy resin, and presents a processing solution in many application areas, including wind turbine blades. Leveraging Cathay Biotech’s line of LCDAs along with their PDA, Cathay Biotech launched ECOPENT® for the engineering materials industry. ECOPENT® provides a renewably sourced polyamide, which is highly desirable for the engineering plastics compounding market. The renewable sources are plant raw materials with a bio-based carbon content of 25% to 100%, which can be analytically verified by third-party laboratories performing ASTM 6866 methodology.
Moreover, bio-based polyamides can be reinforced by fiber composites, such as glass fiber, carbon fiber or other natural fibers. These composites have application in the construction, automotive, and other special equipment areas due to their excellent mechanical properties, which include high specific strength and specific modulus among others. With the introduction of biobased PA5X family composites, and the demonstrated properties of excellent abrasion and impact resistance, easy processing, light weight and recyclability, the possibility of “Replacing Steel with Plastic” and “Replacing Thermoset with Thermoplastic” becomes a distinct reality. For example, the continuous glass/carbon fiber reinforced bio-based PA5X (CFRT-PA5X) has been successfully produced with bio-based polyamide as matrix and continuous glass fiber as reinforcement. In this application, the glass fiber content could be as high as 70%GF. The stretch strength of such unidirectional tape (UD tape) could be higher than 1000MPa due to the high glass fiber content. An important characteristic of these composite laminates with different layer alignments, thickness and size are possible using a hot-forming press. The subsequent glass fiber-based composite laminates show high stretch strength of more than 1000MPa, with maximum tensile modulus up to 45GPa.
Specifically, it has been demonstrated that the 70% continuous glass fiber reinforced E-2260 composite board has a 0° bending strength up to 1,033MPa, which is twice of Polypropylene (PP) composite board with same glass fiber content. The 0° bending modulus also reaches 38MPa, which is 30% higher than that of 70% glass fiber reinforced PP composite board. Furthermore, the 90° bending strength and 0° interlaminar shear strength of such biobased PA5X composite board is 1.5 times of PP composite board with same glass fiber content. Moreover, CFRT-PA5X composites with higher mechanical property could be produced by using continuous carbon fibers instead of glass ones. As an example, the CFRT- ECOPENT® 2260-50% CF has only one-third the density of super-steel but exhibits a 10% higher tensile strength. In addition, the specific strength and specific modulus of CFRT-ECOPENT® 2260-50% CF is 6 times and 2 times of super-steel, respectively. With UD tapes and laminates available, various application scenarios such as dry cargo containers, semitrailers, honeycomb plates and wind turbine blades are all application candidates. Therefore, replacing steel and thermoset with thermoplastic while increasing strength, decreasing weight, and contributing to the goal of carbon neutrality is a real possibility.
References
- Lee HB, Sundberg BN, Sigafoos AN, Clark KJ (2016) Genome engineering with TALE and CRISPR systems in neuroscience. Frontiers in Genetics 7.
- Xiao A, Wu YD, Yang ZP, Hu YY, Wang WY, et al. (2013) EENdb: A database and knowledge base of ZFNs and TALENs for endonuclease engineering. Nucleic Acids Res 41: D415-D422.
- Shapira P, Kwon S, Youtie J (2017) Tracking the emergence of synthetic biology. Scientometrics 112(3):1439-1469.
- Skoog E, Shin JH, Saez-JV, Mapelli V, Olsson L (2018) Biobased adipic acid - The challenge of developing the production host. Biotechnol Adv 36(8): 2248-2263.
- Sajid M, Zhao X, Liu D (2018) Production of 2,5-furandicarboxylic acid (FDCA) from 5-hydroxymethylfurfural (HMF): recent progress focusing on the chemical catalytic routes. Green Chem 20: 5427-5453.
- Yi G, Teong SP, Zhang Y (2015) The direct conversion of sugars into 2,5-furandicarboxylic acid in a triphasic system. ChemSusChem 8(7): 1151-1155.
- Dessbesell L, Souzanchi S, Venkateswara Rao KT, Carrillo AA, Bekker D, et al. (2019) Production of 2,5-furandicarboxylic acid (FDCA) from starch, glucose, or high-fructose corn syrup: techno-economic analysis, Biofuels Bioprod Biorefin 13(5): 1234-1245.
- Yi G, Teong SP, Li X, Zhang Y (2014) Purification of biomass-derived 5-hydroxymethylfurfural and its catalytic conversion to 2, 5-furandicarboxylic acid. Chem Sus Chem 7(8): 2131-2135.
- Ribeiro ML, Schuchardt U (2003) Cooperative effect of cobalt acetylacetonate and silica in the catalytic cyclization and oxidation of fructose to 2, 5-furandicarboxylic acid. Catal Commun 4(2): 83-86.
- Kroger M, Prüße U, Vorlop KD (2000) A new approach for the production of 2, 5- furandicarboxylic acid by in situ oxidation of 5-hydroxymethylfurfural starting from fructose. Top Catal 13: 237-242.
- Oumera AN, Hasana MM, Bahetab AT, Mamata R, Abdullah AA (2018) Bio-based liquid fuels as a source of renewable energy: A review. Renew Sust Energ Rev 88: 82-98.
- Vilela C, Sousa AF, Fonseca AC, Serra AC, Coelho JF, et al. (2014) The quest for sustainable polyesters–insights into the future. Polym Chem 5: 3119-3141.
- Nampoothiri KM, Nair NR, John RP (2010) An overview of the recent developments in polylactide (PLA) research. Bioresour Technol 101(22): 8493-8501.
- Müller HM, Seebach D (1993) Poly (hydroxyalkanoates): A fifth class of physiologically important organic biopolymers? Angew Chem Int Ed Engl 32(4): 477-502.
- Jacquel N, Freyermouth F, Fenouillot F, Rousseau A, Pascault JP, et al. (2011) Synthesis and properties of poly (butylene succinate): Efficiency of different transesterification catalysts. J Polym Sci Part A: Polym Chem 49(24): 5301-5312.
- Dietrich K, Dumont MJ, Del Rio LF, Orsat V (2017) Producing PHAs in the bioeconomy-towards a sustainable bioplastic. Sustain Prod Consum 9: 58-70.
- Alkim C, Cam Y, Trichez D, Auriol C, Spina L, et al. (2015) Optimization of ethylene glycol production from (d)-xylose via a synthetic pathway implemented in Escherichia coli. Microb Cell Factor 14: 127.
- Liu H, Ramos KR, Valdehuesa KN, Nisola GM, Lee WK, et al. (2013) Biosynthesis of ethylene glycol in Escherichia coli. Appl Microbiol Biotechnol 97(8): 3409-3417.
- Pereira B, Li ZJ, Mey DM, Lim CG, Zhang H, et al. (2016) Efficient utilization of pentoses for bioproduction of the renewable two carbon compounds ethylene glycol and glycolate. Metab Eng 34: 80-87.
- Pereira B, Zhang H, De Mey M, Lim CG, Li ZJ, et al. (2016) Engineering a novel biosynthetic pathway in Escherichia coli for production of renewable ethylene glycol. Biotechnol Bioeng 113(2): 376-383.
© 2022 Xiucai Liu. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






