- Submissions

Full Text
Polymer Science: Peer Review Journal
The Potential of Hermetia Illucens as a Source of Chitin, Chitosan and their Melanin Complexes
Khayrova A* and Lopatin S
Research Centre of Biotechnology of the Russian Academy of Sciences, Russia
*Corresponding author: Khayrova A, Research Centre of Biotechnology of the Russian Academy of Sciences, Leninsky prospect, Moscow, Russia
Submission: March 18, 2022;Published: May 10, 2022

ISSN: 2770-6613 Volume3 Issue4
Abstract
Interest in insects as a source of valuable biologically active substances has significantly increased over the past few years. Insects serve as an alternative source of chitin, which forms up to 40% of their exoskeleton. Chitosan, a deacetylated derivative of chitin, attracts the attention of scientists due to its unique properties (sorption, antimicrobial, film-forming, wound healing). Furthermore, some insect species such as Hermetia illucens are unique and can be used to obtain chitin- and chitosan-melanin complexes in the later stages of ontogenesis. Due to the synergistic effect, chitosan and melanin can enhance each other’s biological activity, providing a wide range of potential applications.
Keywords:Chitin; Chitosan; Melanin; Complex; Insect; Black soldier fly; Hermetia Illucens
Introduction
Chitin and chitosan: properties, sources and applications
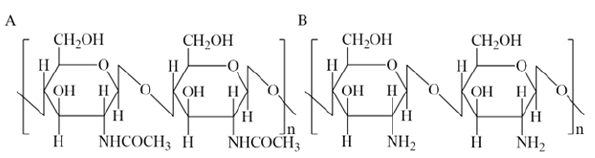
Chitin and chitosan are linear polysaccharides that consist of varying amounts of N-acetyl- 2-amino-2-deoxy-D-glucopyranose and 2-amino-2-deoxy-D-glucose in pyranose form and are linked to each other by 1-4 glycosidic bonds [1] (Figure 1). Chitin was first described by the French chemist Henri Braconnot in 1811, and the name “chitin” comes from the Greek word “chiton”, which means covering [2]. Chitin is the second most common biopolymer after cellulose, containing approximately 2-4% of 2-amino-2-deoxy-D-glucose residues [1]. Chitosan is obtained by chemical, less often enzymatic, deacetylation of chitin. Chitosan is conventionally considered to be a polymer soluble in dilute organic acids, which corresponds to a degree of deacetylation of about 40-45% [3,4]. Chitin is found in invertebrates, crustacean shells or insect cuticles, in the cell walls of fungi, green algae, and yeast [5,6]. In fact, crustacean shells contain 30-40% protein, 30-50% calcium carbonate and phosphate, and 20-30% chitin [7,8]. However, chitin content varies depending on the source or even the species from which it is isolated. For example, the shells of Crangon crangon shrimp may contain 10-38% protein, 31- 44% minerals, and 24-46% chitin [9]. In fungi, chitin is present in the form of a chitin-glucan complex, which is extremely difficult to separate into individual polysaccharides [10]. The main difference between insects as a source of chitin is the high content of melanin covalently bound to the target biopolymer. To remove melanin, a bleaching step can be introduced. However, discoloration (bleaching) does not remove the pigment completely, only weakening the colour. Chitosan is the most important chitin derivative. Chitosan can easily undergo structur-al modifications due to the presence of many functional groups on its polysaccharide chain, primarily amino groups [11]. Therefore, chitosan has an important property – solubility in weakly acidic aqueous solutions [12-14]. Amino groups in the structure of chitosan are chelating ligands capable of binding various metal ions.
Figure 1:Chemical structure of chitin (A) and chitosan (B).
The degree of deacetylation (DD) and the molecular weight of chitosan strongly affect many physicochemical, such as solubility, hydrophilicity, and crystallinity, as well as biological properties of chitosan. Various analytical methods are used to determine DD, including IR spectroscopy, pyrolysis gas chromatography, gel permeation chromatography and UV-visible spectrophotometry, 1H NMR spectroscopy, 13C solid state NMR, thermal analysis, various titration schemes, acid hydrolysis, HPLC, methods of separation spectrometry and near-infrared spectroscopy [15]. Chitosan offers a wide range of applications including biotechnology, food processing and medicine. The biopolymer is widely used in chemical and textile industries, membranes, and wastewater treatment due to its polycationic nature [11,12,16,17]. In addition, it plays a significant role in agriculture, cosmetics, food protection, papermaking, and tissue engineering [17-20].
Methods for obtaining chitin
Crustacean shells are the main source of raw materials for chitin production. The method for chitin production includes deproteinization and demineralization to remove proteins and inorganic calcium carbonate together with small amounts of pigments and fat [21]. In some cases, an additional bleaching step is used to get rid of residual pigments. Over the past decades, various methods have been proposed to obtain crystalline chitin; however, the standard method has not yet been defined. The deproteinization step involves the breaking of chemical bonds between chitin and proteins. This process is carried out heterogeneously using chemicals that can depolymerize the biopolymer. Attempts were made to replace chemical deproteinization with the enzymatic one [22], but it did not result in complete removal of proteins. Demineralization is carried out to remove minerals, primarily calcium carbonate. This step usually includes treatment with acids such as HCl, HNO3, H2SO4, CH3COOH, and HCOOH [23,24]. Among these acids, dilute hydrochloric acid is preferred. Chitin can be obtained in amorphous form when its crystal lattice is destroyed. Colloidal chitin is obtained by precipitation of the biopolymer from its solutions in mineral acids (hydrochloric, aqueous sulfuric, phosphoric, or methane sulfonic acids) [25-27]. A colloidal solution of chitin is used as a substrate for determining the activity of chitinolytic enzymes and lysozyme.
Chitin plays an important role in the structure of insects: the growth and development of insects strictly depend on the ability to reconstruct chitinous structures [28]. The biopolymer is present in insects at all stages of ontogenesis. Chitin is the main component of the insect cuticle. Physicochemical analysis shows that the chitin content is up to 40% of the dry mass of the cuticle, depending on the insect species and varies significantly with the type of cuticle, even in one organism [29]. For instance, Holotrichia parallela contains 15% chitin [30], Bombyx mori – 15-20% [31], and cicada – 36% [32]. Chitin is found in the exo- and endocuticle, in the newly secreted, necrotic procuticle, but not in the epicuticle, the outermost part of the integument [33]. It acts as a light, but at the same time mechanically strong frame material and is always associated with cuticle proteins, which frequently determine the mechanical properties of the cuticle. In insects, chitin is associated with melanin, which is not found in crustaceans. In addition, insects may contain a large percentage of fat. As a result, additional purification steps are introduced in chitin production. Despite these issues, the number of studies on the extraction of chitin and its derivatives from insects has been growing lately. This applies not only to large-scale insect rearing, but also to exotic species.
Melanins
Melanins (from the Greek melanos, which means “dark”) are one of the most mysterious biopolymers in the biosphere, despite their wide distribution in nature [34]. These biopolymers are synthesized by oxidation and polymerization of phenolic/indolic precursors. The degree of pigmentation is largely determined by the ability of specialised cells to synthesize brown-black eumelanin and yellow reddish pheomelanin [35]. Eumelanin is generally considered to be a heterogeneous macromolecule of 5,6-dihydroxyindole and 5,6-dihydroxyindole-2-carboxylic acid, its 2-carboxylated form. Pheomelanin is derived from the sulfur-containing cysteinyl dopa and is believed to be a heterogeneous macromolecule. The function of melanin is determined by its physical and chemical properties. These properties − antioxidant and free absorption of radicals, broadband ultraviolet and visible absorption, and strong relaxation of photo-excited electronic states − are affected by the structure of the molecular, supramolecular, and aggregate level [36,37]. Additionally, melanin is characterised by a strong negative charge, high molecular weight, and hydrophobic nature [38]. Melanin is a photoprotective pigment. The protective effect of melanin is due to its high efficiency in absorbing and scattering photons, especially photons with higher energy from the ultraviolet and blue part of the solar spectrum. The energy of absorbed photons is quickly and efficiently converted into heat as a result of ultrafast photodynamic [39]. It is likely that the photoprotective properties of melanin are also due to its ability to neutralize the excited states of certain molecules and remove reactive oxygen species that can form in pigmented cells. Melanin has been shown to be a potent antioxidant in various model systems [40].
In addition to the variety of biological functions, this biopolymer has many potential applications in biophysics, materials science, cosmetics, and healthcare [41,42]. The capability of melanin is determined not only by its chemical composition at the molecular level, but also by its versatility as a component of the supramolecular structure in the cellular environment.
Obtaining of Chitin, Chitosan and their Melanin Complexes from Hermetia Illucens
Figure 2: Life cycle diagram of black soldier fly (Hermetia illucens).
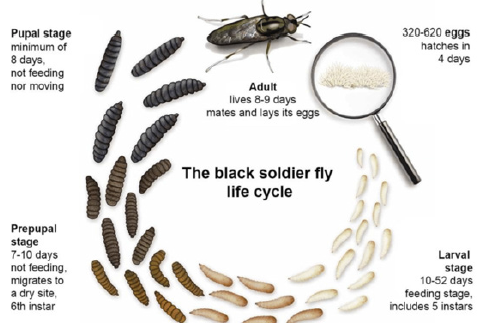
The black soldier fly Hermetia illucens is found in nutrient-rich environments in the western part of our planet. The larvae of these insects are reared on many types of decaying organic materials and are used for composting, as they can convert organic waste into nutrient- rich fertilizer. The larvae are able to process a wide range of substrates, including agricultural by-products and organic waste of animal or plant origin [43]. This offers opportunities for an innovative technology of waste bioconversion by insects [44]. In addition, black soldier fly larvae are a great source of fat and protein for animal feed and biodiesel production [45]. A number of companies around the world, including South Africa, Canada, USA, Netherlands, China and Russia, are currently incorporating the technology of Hermetia illucens rearing to solve ecological issues. Black soldier fly is available at all stages of ontogenesis, unlike other industrially reared insect species, such as bees and silkworms (Figure 2). At earlier stages, Hermetia illucens larvae are unpigmented, and are a source of chitin and chitosan. At later stages of development – prepupae, pupae, corpses – insects accumulate melanin, which can easily be observed. Hence, they become a source of both chitin- and chitosan-melanin complexes. Additionally, chitin and melanin in Hermetia illucens are linked by strong covalent bonds, which distinguishes them from the bee corpses, where the complexes are obtained by mixing the biopolymers together.
Known methods for producing chitin from Hermetia illucens are based on a standard approach involving sequential removal of impurities from the starting material. Table 1 summarises a number of studies devoted to the extraction of chitin, chitosan and their melanin complexes from Hermetia illucens. Waśko et al. [46] have reported the physicochemical structure of chitin isolated from Hermetia illucens. First, the samples consisting of pupal exuviae, and corpses were cleaned, dried to constant weight, and ground in a laboratory mill. Demineralization was carried out using 1 M HCl for 1h. The demineralized powder was washed with distilled water. Deproteinization was performed using 1 M NaOH at 80 ℃ for 24h. The extract was filtered and decolorized using 1% KMnO4. Excess KMnO4 was removed with 4% oxalic acid. The white-grey final product was then filtered, washed with distilled water and dried. In that study, the chitin-containing raw material is highly pigmented, and some of the melanins are covalently bound to chitin. Therefore, a bleaching step was added, carried out in the presence of a 1% KMnO4 solution. However, this treatment does not remove the melanin completely. In addition, the defatting step was not carried out, and potential impurities (protein, fat, melanin) in the obtained chitin were not determined. The degree of acetylation of the obtained chitin samples, based on elemental analysis data, was equal to 250% and 179%, which exceeded the theoretical maximum of 100%. This indicates the presence of impurities in chitin. Caligiani et al. [47] have employed an alternative method for obtaining chitin from the prepupae. The starting material contained (dry weight) 32% protein, 37% fat, 19% minerals and 9% chitin. Defatting was carried out in two steps using petroleum ether. The defatted material was then treated with 1M NaOH solution, and demineralization was carried out using 2N HCl. After centrifugation, the precipitate was washed with water and dried. As a result, it was possible to fractionate the starting material into three main products – fat, protein and chitin. The chitin yield varied from 11.7% to 14.6%, depending on the degree of acetylation. However, the study does not provide data confirming the purity of the obtained chitin. D’Hondt et al. [48] extracted chitin from the larvae through the series of steps: washing with distilled water, deproteinization with 1 M NaOH solution at 80 ℃ for 1h and washing with distilled water until neutral pH. Acidic hydrolysis was carried out in heat- and pressure-resistant glass jars. 5ml of 3 or 6N HCl was added to 50mg of dry, crushed and demineralized samples. The reaction was carried out at 110 ℃ for 2-16h. The reaction mixture was then centrifuged to remove residual insoluble material, followed by filtration. Based on the above information, it can be concluded that applied conditions are too strong, while there is no defatting present.
Table 1: H. illucens as a source of chitin, chitosan and their melanin complexes. *not available.

Hahn et al. [49] described the optimal conditions for obtaining chitosan from the larval exoskeletons and evaluated the final product relative to crab chitosan. The influence of the variables of deproteinization was analysed using linear regression model; the chitin content in the deproteinized material was found to be 83% and 87% on a small and large scales, respectively. Two types of deacetylation reactions were carried out. Heterogeneous deacetylation at 120 ℃ led to DD of 72% and a maximum yield of 43% with respect to chitin. Homogeneous deacetylation at 4 ℃ led to DD of 34% and a low chitosan yield of 13%. However, chitosan showed excellent film-forming properties and high viscosity when dissolved in acetic acid. The results of the study confirmed that insect chitosan has properties comparable to chitosan from crustaceans, which are highly dependent on the conditions used for production. Wang et al. [50] compared the physicochemical properties of the chitinous matrix obtained at different stages of insect development: larvae, prepupae, pupae, and imagos. The chitin content was 3.6%, 3.1%, 14.1% and 2.9%, respectively. The crystallinity index increased from larva to adult flies: 33.1%, 35.1%, 68.4% and 87.9%, respectively. However, the authors do not take into account the presence of melanin at the later stages of insect development, starting with the pupae. In the study by Nafisah et al. [51], the larvae were defatted in a hexane solution for 6h. Chitin was extracted by the method [52], however, the exact method (in an open or closed system) was not specified. Demineralization took place in 1M HCl at 100 ℃ for 20min, and deproteinization – in 1M NaOH at 80 ℃ for 24h. Then, the samples were treated with 0.4% Na2CO3 several times and dried. Chitin was deacetylated with 40% NaOH solution, while NaBH4 was used as a reducing agent. Impurities were identified at each treatment step: the protein content in the larvae was approximately 43.0%, chitin – 20.3%.
It can be concluded that the black soldier fly serves as a source of pure chitin and chitosan only at the early stages of its development, including the 5th instar larvae. At the later stages of ontogenesis (prepupae, pupae, imagos), melanin is formed in insects, which is strongly bound to chitin. Discoloration of such complex does not lead to the breaking of these bonds or complete removal of melanin; therefore, pure chitin cannot be obtained at later stages. The process of obtaining the chitosan-melanin complex from Hermetia illucens is discussed by Bastrakov et al. [53]. Nevertheless, the method itself is not described, the authors refer to the work [54], which indicates that the complexes are a mixture of biopolymers. Pigments were identified based on the assessment of their solubility, spectral and paramagnetic properties, which also indicates that melanin was obtained separately and then added to chitosan. In another study by Ushakova et al. [55], melanin and chitin-melanin complexes were obtained from corpses. Water-soluble melanin was extracted using 10% NaOH at room temperature for 16h. Melanin was obtained by adding concentrated HCl, washed with distilled water to neutral pH, centrifuged at least 3 times, and dried. The chitin-melanin complex was obtained by acid hydrolysis using 25% H2SO4 under reflux for 3h. Then, the hydrolysate was cooled to 40 ℃, filtered, washed with distilled water and dried. The complex yield was equal to 28%. At the end, the defatting step using chloroform was carried out for both the complex and the corpses. However, neither protein/fat content nor the purity of the final product were determined. Khayrova et al. [56] have examined the production of both low- and high-molecular-weight chitosan from larvae, the unpigmented starting material. Amorphous chitin was obtained using the direct extraction method [57]. The yield of amorphous chitin was 7%.
The deacetylation reaction of amorphous chitin was carried out in 30% NaOH in a water bath at 100 ℃ for 1h. The reaction mixture was subsequently diluted with water, and the excess alkali was removed using a cation exchange resin. The solution was dialyzed against water and lyophilized. The chitosan yield was 32%. A defatting step using a mixture of CHCl3: CH3OH (7:3) was added to obtain crystalline chitin. Demineralization was carried out with 2% HCl at 20 ℃ for 2h. Deproteinization was implemented by treatment with 5% NaOH in a water bath at 50 ℃ for 2h. The obtained chitin was filtered, washed with distilled water until neutral pH, and lyophilized. The chitin yield was 46%. The deacetylation reaction was carried out using 50% NaOH at 100 ℃ for 2h. The chitosan yield was 80%. In both cases the products were characterised by HPLC, 1H NMR and conductometric titration. Deacetylation was followed by destruction of amorphous chitin, resulting in low molecular weight chitosan (15kDa, DD of 42-47%). The molecular weight of chitosan obtained by the second method was 160kDa, and DD – 90%. In other experiments by Khayrova et al. [58], a method for obtaining chitin- and chitosan-melanin complexes from pupal exuviae and corpses was developed. The chitin-containing raw material was characterised at each treatment step, the content of impurities (protein, fat) was determined, which was not previously observed in other works. The fat in corpses differed due to the presence of wax, thus, a defatting step with diethyl ether was introduced. Demineralization was carried out using 1% HCl at 20 ℃ for 2h. The solid residue was then filtered, washed with distilled water to neutral pH values, and lyophilized. The chitin-melanin complex was obtained by treatment with 30% NaOH in a water bath at 50 ℃ for 2h. Then, the resulting solid residue was separated on a porous glass filter, washed with water until neutral pH, and lyophilized.
The chitosan-melanin complex was obtained by treatment with 50% NaOH in a 100 ℃ water bath for 2h, stirring occasionally. The suspension was cooled, the precipitate was separated on a porous glass filter, washed with distilled water until neutral pH, and lyophilized. Lin et al. [59] developed a method to extract chitin from spent pupal shell by the microbial fermentation using Bacillus lichenformis A6. The recovery rate of chitin content by the microbial fermentation method was equal to 12.4%. The structures of BSF chitin and chitosan were further characterised by FTIR, XRD, and SEM, and the chitin obtained from BSF was observed in α form. The crystalline index values of chitin and chitosan were 52.8% and 55.4%, respectively. The surface morphology was examined by SEM, revealing nanofiber structures. Złotko et al. [60] presented various procedures for the isolation of chitin from Hermetia illucens pupal exuviae. The obtained chitin variants were characterised using different techniques such as optical and confocal microscopy, FTIR, XRD, EDX, thermogravimetric analysis. The tested chitin isolated with an efficiency of 5.69–7.95% was the form with a crystallinity degree of 60% and maximum degradation temperature of 392 ℃. Furthermore, the nickel ion biosorption process on chitin was characterised, and the mechanism of this process to be ion exchange and complexation was proposed. The study showed the potential of Hermetia illucens chitin as a metal bio sorbent that can be obtained with relatively high efficiency and strong sorption properties.
Conclusion
In this review, Hermetia illucens was considered as a new source for production of chitin, chitosan, and their melanin complexes. The unique properties of melanin pigments, based on their stable free radical state, can be significantly improved and expanded when complexed with chitosan and its derivatives. Chitin and chitosan are biopolymers possessing a number of highly valuable characteristics (radioprotectors, antioxidants, bactericides, fungicides, chelators, complexing agents, hepatoprotections, etc.) that complement the biological activity of melanins. Thus, it can be concluded that the unique photo- and radioprotective properties of melanin pigments, in combination with chitosan, will allow to obtain composites with new, enhanced biological activities which could potentially be applied in various fields.
Acknowledgement
The authors declare no conflict of interest. No ethical approval required. No informed consent required. The research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Mihailov S and Varlamov V (2013) Chitosan – biopolymer with unique properties. In: Skryabin K, Mihailov S, Varlamov V (Eds.), Chitosan. Centre of Bioengineering RAS, Moscow, Russia, pp. 5-18.
- Elieh-Ali-Komi D, Hamblin MR (2016) Chitin and chitosan: Production and application of versatile biomedical nanomaterials. Int J Adv Res 4(3): 411-427.
- Croisier F, Jérôme C (2013) Chitosan-based biomaterials for tissue engineering. Eur Polym J 49: 780-792.
- Gonil P, Sajomsang W (2012) Applications of magnetic resonance spectroscopy to chitin from insect cuticles. Int J Biol Macromol 51(4): 514-522.
- Berger RT, Christina A, Miranda P, Pessoa MA, Barbosa D, et al. (2018) Chitosan produced from mucorales fungi using agroindustrial by-products and its efficacy to inhibit colletotrichum Int J Biol Macromol 108: 635-641.
- Kannan M, Nesakumari M, Rajarathinam K, Singh A (2010) Production and characterization of mushroom chitosan under solid-state fermentation conditions. Adv Biol Res 4(1): 10-13.
- Kumari S, Rath P, Sri Hari Kumar A, Tiwari TN (2015) Extraction and characterization of chitin and chitosan from fishery waste by chemical method. Environ Technol Innov 3: 77-85.
- Kumirska J, Czerwicka M, Kaczyński Z, Bychowska A, Brzozowski K, et al. (2010) Application of spectroscopic methods for structural analysis of chitin and chitosan. Mar Drugs 8(5): 1567-1636.
- Bajaj M, Winter J, Gallert C (2011) Effect of deproteination and deacetylation conditions on viscosity of chitin and chitosan extracted from Crangon crangon shrimp waste. Biochem Eng J 56(1-2): 51-62.
- Hong Y, Ying T (2019) Characterization of a chitin-glucan complex from the fruiting body of Termitomyces albuminosus (Berk.) Heim. Int J Biol Macromol 134: 131-138.
- Wang Y, Wang E, Wu Z, Li H, Zhu Z, et al. (2014) Synthesis of chitosan molecularly imprinted polymers for solid-phase extraction of methandrostenolone. Carbohydr Polym 101: 517-523.
- Al-Manhel AJ, Al-Hilphy ARS, Niamah AK (2018) Extraction of chitosan, characterisation and its use for water purification. J Saudi Soc Agric Sci 17(2): 186-190.
- Shajahan AS, Shankar A, Sathiyaseelan KS, Narayan V, Narayanan V, et al. (2017) Comparative studies of chitosan and its nanoparticles for the adsorption efficiency of various dyes. Int J Biol Macromol 104: 1449-1458.
- Bonilla J, Fortunati E, Atarés L, Chiralt A, Kenny JM (2014) Physical, structural and antimicrobial properties of poly vinyl alcohol-chitosan biodegradable films. Food Hydrocoll 35: 463-470.
- Kumar MNVR (2000) A review of chitin and chitosan applications. React Funct Polym 46(1): 1-27.
- Niederhofer A, Müller BW (2004) A method for direct preparation of chitosan with low molecular weight from fungi. Eur J Pharm Biopharm 57(1): 101-105.
- Zahra J, Jafar A (2012) Chitosan: A brief review on structure and tissue engineering application. Tissue Eng Res 1: 8-11.
- Hijazi N, Rodier E, Letourneau JJ, Louati H, Sauceau M, et al. (2014) Chitosan nanoparticles generation using CO2 assisted processes. J Supercrit Fluids 95: 118-128.
- Muxika A, Etxabide A, Uranga J, Guerrero P, de la Caba K (2017) Chitosan as a bioactive polymer: processing, properties and applications. Int J Biol Macromol 105(2): 1358-1368.
- Bano I, Arshad M, Yasin T, Ghauri MA, Younus M (2017) Chitosan: A potential biopolymer for wound management. Int J Biol Macromol 102: 380-383.
- Younes I, Rinaudo M (2015) Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar Drugs 13(3): 1133-1174.
- Melnikov V, Melnikov A, Yarigin Y, Shelepov V, Motovilov O, et al. (2014) Method for obtaining a chitin-mineral complex from shell-containing waste from gammarus processing. Russian Patent 2: 541-645.
- No HK, Hur EY (1998) Control of foam formation by antifoam during demineralization of crustacean shell in preparation of chitin. J Agric Food Chem 46(9): 3844-3846.
- Percot A, Viton C, Domard A (2003) Optimization of chitin extraction from shrimp shells. Biomacromolecules 4(1): 12-18.
- Karrer P, Hofmann A (1929) Polysaccharide XXXIX. Über den enzymatischen abbau von chitin und chitosan I. Helv Chim Acta 12(1): 616-637.
- Jeuniaux C (1966) Chitinases. Methods Enzymol 8: 644-650.
- Leger RJS, Cooper RM, Charnley AK (1986) Cuticle-degrading enzymes of entomopathogenic fungi: Regulation of production of chitinolytic enzymes. J Gen Microbiol 132(6): 1509-1517.
- Merzendorfer H, Zimoch L (2003) Chitin metabolism in insects: structure, function and regulation of chitin synthases and chitinases. J Exp Biol 206(24): 4393-4412.
- Kramer KJ, Hopkins TL, Schaefer J (1995) Applications of solids NMR to the analysis of insect sclerotized structures. Insect Biochem Mol Biol 25(10): 1067-1080.
- Liu S, Sun J, Yu L, Zhang C, Bi J, et al. (2012) Extraction and characterization of chitin from the beetle Holotrichia parallela Molecules 17: 4604-4611.
- Zhang M, Haga A, Sekiguchi H, Hirano S (2000) Structure of insect chitin isolated from beetle larva cuticle and silkworm (Bombyx mori) pupa exuvia. Int J Biol Macromol 27(1): 99-105.
- Sajomsang W, Gonil P (2010) Preparation and characterization of α-chitin from cicada sloughs. Mater Sci Eng 30(3): 357-363.
- Andersen SO (1979) Biochemistry of insect cuticle. Annu Rev Entomol 24: 29-59.
- Shosuke I, Kazumasa W, Marco DI, Alessandra N, Alessandro P (2011) Structure of melanins. In: Borovansky J, Riley PA (Eds.), Melanins and melanosomes: Biosynthesis, biogenesis, physiological, and pathological functions. Wiley-VCH Verlag & Co, Weinheim, Germany, pp. 167-185.
- Meredith P, Sarna T (2006) The physical and chemical properties of eumelanin. Pigment Cell Res 19(6): 572-594.
- Riesz J, Gilmore J, Meredith P (2006) Quantitative scattering of melanin solutions. Biophys J 90(11): 4137-4144.
- Cordero RJB, Robert V, Cardinali G, Arinze ES, Thon SM, et al. (2018) Impact of yeast pigmentation on heat capture and latitudinal distribution. Curr Biol 28(16): 2657-2664.
- White LP (1958) Melanin: A naturally occurring cation exchange material. Nature 182(4647): 1427-1428.
- Ye T, Simon JD (2003) Comparison of the ultrafast absorption dynamics of eumelanin and pheomelanin. J Phys Chem 107(40): 11240-11244.
- Sarna T, Swartz HN (2006) The physical properties of melanin. In: Nordlund JJ, Boissy RE, Hearing VJ, King RA, Oetting WS, (Eds.), The pigmentary systems: Physiology and pathophysiology. Blackwell Publishing Ltd, Oxford, United Kingdom. pp. 311-341.
- d'Ischia M, Napolitano A, Pezzella A, Meredith P, Sarna T (2009) Chemical and structural diversity in eumelanins: unexplored bio-optoelectronic materials. Angew Chem Int Ed Engl 48(22): 3914-3921.
- Panzella L, Ebato A, Napolitano A, Koike K (2018) The late stages of melanogenesis: exploring the chemical facets and the application opportunities. Int J Mol Sci 19(6): 1753.
- Hoc B, Noël G, Carpentier J, Francis F, Caparros MR (2019) Optimization of black soldier fly (Hermetia illucens) artificial reproduction. PLoS ONE 14: e0216160.
- Čičková H, Newton GL, Lacy RC, Kozánek M (2015) The use of fly larvae for organic waste treatment. Waste Manag 35: 68-80.
- Li Q, Zheng L, Cai H, Garza E, Yu Z, et al. (2011) From organic waste to biodiesel: Black soldier fly, Hermetia illucens, makes it feasible. Fuel 90(4): 1545-1548.
- Waśko A, Bulak P, Polak-BM, Nowak K, Polakowski C, et al. (2016) The first report of the physicochemical structure of chitin isolated from Hermetia illucens. Int J Biol Macromol 92: 316-320.
- Caligiani A, Marseglia A, Leni G, Baldassarre S, Maistrello L, et al. (2018) Composition of black soldier fly prepupae and systematic approaches for extraction and fractionation of proteins, lipids and chitin. Food Res Int 105: 812-820.
- D'Hondt E, Soetemans L, Bastiaens L, Maesen M, Jespers V, et al. (2020) Simplified determination of the content and average degree of acetylation of chitin in crude black soldier fly larvae samples. Carbohydr Res 488: 107899.
- Hahn T, Roth A, Ji R, Schmitt E, Zibek S (2020) Chitosan production with larval exoskeletons derived from the insect protein production. J Biotechnol 310: 62-67.
- Wang H, Rehman KU, Feng W, Yang D, Rehman RU, et al. (2020) Physicochemical structure of chitin in the developing stages of black soldier fly. Int J Biol Macromol 149: 901-907.
- Nafisah A, Nahrowi, Mutia R, Jayanegara A (2019) Chemical composition, chitin and cell wall nitrogen content of Black Soldier Fly (Hermetia illucens) larvae after physical and biological treatment. IOP Conf Ser.: Mater Sci Eng 546: 042028.
- Paulino AT, Simionato JI, Garcia JC, Nozaki J (2006) Characterization of chitosan and chitin produced from silkworm crysalides. Carbohydr Polym 64(1): 98-103.
- Bastrakov AI, Dontsov AE, Ushakova NA (2016) Black soldier fly Hermetia illucens under in vitro breeding as a renewable source of melanin-chitosan complex. Proceedings of the RAS Ufa Scientific centre, 4: 77-79.
- Pogarskaya NV, Selionova MI, Binatova VV (2008) Production of chitosan-melanin complex from dead bees and identification of its physicochemical and biological characteristics. Vetkorm 6: 28-29.
- Ushakova N, Dontsov A, Sakina N, Bastrakov A, Ostrovsky M (2019) Antioxidative properties of melanins and ommochromes from black soldier fly Hermetia illucens. Biomolecule 9(9): 408.
- Khayrova A, Lopatin S, Varlamov V (2019) Black soldier fly Hermetia illucens as a novel source of chitin and chitosan. Int J Sci 8: 81-86.
- Khayrova AS, Lopation SA, Sinitsyna OA, Sinitsyn AP, Varlamov VP (2018) Obtaining chitin from the black soldier fly Hermetia illucens by direct extraction. Proceedings of the RAS Ufa Scientific centre 3: 84-87.
- Khayrova A, Lopatin S, Varlamov V (2020) Obtaining chitin/chitosan-melanin complexes from black soldier fly Hermetia illucens. IOP Conf Ser: Mater Sci Eng 809: 012020.
- Lin YS, Liang SH, Lai WL, Lee JX, Wang YP, et al. (2021) Sustainable extraction of chitin from spent Pupal shell of black soldier fly. Processes. 9(6): 976.
- Złotko K, Waśko A, Kamiński DM, Budziak-WI, Bulak P, et al. (2021) Isolation of chitin from black soldier fly (Hermetia illucens) and its usage to metal sorption. Polymers 13(5): 818.
- Khayrova A, Lopation S, Shagdarova B, Sinitsyna O, Sinitsyn A, et al. (2022) Evaluation of antibacterial and antifungal properties of low molecular weight chitosan extracted from Hermetia illucens relative to crab chitosan. Molecules 27(2): 577.
© 2022 Khayrova A. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






