- Submissions

Full Text
Polymer Science: Peer Review Journal
The Usage of PEG in Drug Delivery Systems- A Mini Review
Özer S and Yenilmez E*
Department of Pharmaceutical Technology, Faculty of Pharmacy, Anadolu University, Turkey
*Corresponding author:Yenilmez E, Department of Pharmaceutical Technology, Faculty of Pharmacy, Anadolu University, Eskişehir, Turkey
Submission: April 05, 2022;Published: April 19, 2022

ISSN: 2770-6613 Volume3 Issue4
Abstract
Designing polymeric delivery systems for drugs and genes to implement individual biological functions is an area of continuing interest. Poly (Ethylene Glycol) (PEG) is one of the most successfully adopted materials for designing this kind of drug delivery systems and it exhibits a controllable and sustained release profile. Due to its unique structure, PEG molecules are used to reduce the immunogenicity of drug delivery systems and active substances and to avoid unwanted enzymolysis. PEG based copolymers also play a very important role as biomedical materials due to their biocompatibility, biodegradability and easily controllable characters. This mini review summarizes the state-of-the-art use of PEG in therapeutic applications.
Keywords:Polyethylene glycol; Drug delivery; Hydrogel
Abbreviations:PEG: Polyethylene Glycol; FDA: Food and Drug Administration; BSA: Bovine Serum Albumin
Introduction
Polymers, which are formed by the formation of larger molecules by establishing bonds between simple structures called monomers, have recently become a frequent subject of research in the field of medicine and biotechnology [1]. These high molecular weight substances, which are formed because of the combination of the word’s “poly” used to mean “many” in Greek and “meros” used to mean “part/section”, are called “Polymer” [2,3]. Natural polymers, like synthetic polymers, consist of simple and repetitive parts, and new polymers synthesized because of development studies on natural polymers are called semi-synthetic polymers [4]. When the idea of using water- soluble polymers for innovative drug delivery systems, particularly parenteral systems, was first proposed, the industry viewed the idea as an impractical and very risky scientific curiosity, then because of studies on polymers, these high molecular weight molecules began to take place in controlled drug release systems as rate-controlled membranes or biodegradable implants [5-7]. PEG is one of the most important of these water-soluble polymers. After the discovery of PEG in the 1950s, the first pegylation process took place in the late 1970s and thus PEG gained an important place in drug delivery systems [8]. Besides the high water- soluble nature of PEG, it is a non-toxic, non- immunogenic and non-antigenic and FDA- approved conjugate. Therefore, PEG is frequently preferred in drug delivery systems [9,10]. Also, the trend towards biotechnological drugs in recent decades shows that PEG will take place more in drug delivery systems in the future [11].
Polyethylene glycol (PEG)
The emergence of PEG chemistry began in 1977 with the findings of Abuchowski et al. reported that alteration of the immunological properties of Bovine Serum Albumin (BSA) was achieved by the covalent attachment of poly(ethylene) glycol, more appropriately termed PEG [12]. Polyethylene Glycols (PEGs) are polymers of ethylene oxide with the generalized formula HO (CH2_CH2_O)n-H, and “n” indicating the average number of oxyethylene groups and are composed of polyether compounds repeating ethylene glycol units according to the constituent monomer or parent molecule (Figure 1) [13,14]. They are FDA approved synthetic polymers used in food, cosmetics and pharmaceutical industries in non-toxic, white solid form, it is soluble in water, most organic solvents and aromatic hydrocarbons, but slightly soluble in aliphatic hydrocarbons [15,16]. Apart from its use in drug delivery systems in pharmacy, it is used as a water-soluble lubricant in rubber molds, textile fibers and metal forming processes. It is also commonly used in foodstuffs and their packaging, hair preparations and cosmetic preparations. It is also used as a stationary phase in gas chromatography. PEG content is also found in watercolors, paper coatings, lacquers and ceramics [17-19].
Figure 1:Polymerization of ethylene glycol [14].
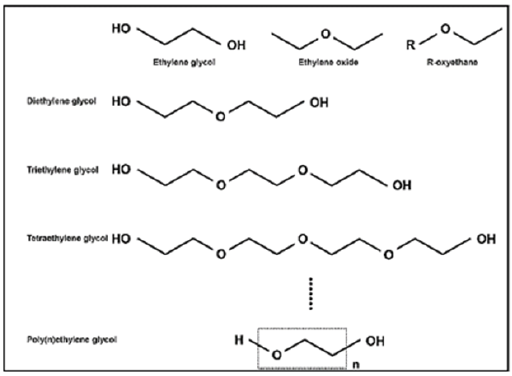
Figure 2:Controllable and sustainable antidiabetic drug delivery systems [29].
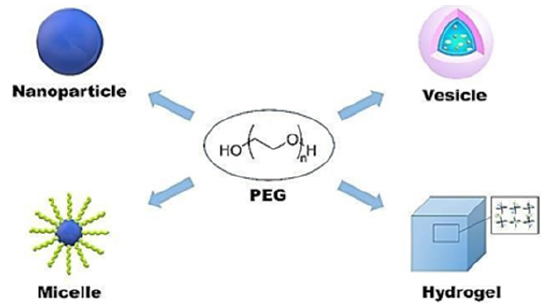
Many fundamental studies have revealed the structural properties of PEG-based hydrogels, such as swelling properties, mechanical properties, and molecular transport properties. Based on these studies, PEG-based controlled release systems containing large biomacromolecules such as nucleic acids, peptides and proteins have been prepared from small molecular weight drugs [20-25]. Two important considerations in the design of PEG hydrogels are stability and bioavailability. To obtain the desired therapeutic efficacy from a designed drug, PEG hydrogels must be stable and capable of releasing the correct dosage of activ substance [26-28]. The release of an active substance from PEGbased hydrogels depends on multiple factors such as the method of loading of the drug, its size, molecular properties, the dose required for administration, and the release profile [30-32]. The most important feature of PEG that enables it to be used in controlled release systems is its protein resistance (Figure 2) [33-35]. Due to the hydrophilic nature of PEG, the polymer chain is firmly attached to hydrogen bonds in water, which inhibits or inhibits protein adsorption to the PEG chain [36-38]. Due to this feature, PEG chains on the surface prolong activities such as endocytosis, phagocytosis, liver effects and adsorptive processes in the body, allowing the therapeutic proteins to bound to it to remain in the circulation for a longer time [41-42].
PEGylation in Drug Delivery
PEGylation describes the modification of a protein, peptide, or non-peptide molecule by the attachment of one or more Polyethylene Glycol (PEG) chains [31,43,44]. PEG-drug conjugates have some advantages, prolonged residence in the body, a reduced degradation by metabolic enzymes and reduction or elimination of protein immunogenicity. Due to these properties, PEGylation plays a very important role in drug delivery today, increasing the potential of peptides as well as proteins as therapeutic agents. There are PEGylated pharmaceutical products on the market today and they play a very important role in the drug delivery system [9,30,43]. From 1995 to 2022, 27 macromolecules, 2 small molecules and 1 nanoparticulate drug have been approved by The United States Food and Drug Administration (FDA) and in addition, Comiranty® (COVID-19 vaccine, mRNA by Pfizer/BioNTech) in which PEGylation technology was used in the rapid development of mRNA-based COVID-19 vaccine received full approval by the FDA in August 2021 [44,45].
Conclusion
Thanks to its unique structure, PEG provides enhanced physicochemical properties and biodegradability to natural or artificial materials. Drug delivery systems composed of PEG can protect drugs with PEGs long hydrophilic chains. And this property prolongs internal life, and promote the stability of particles, leading to improved therapeutic effects. Therefore, the expected effect from the drug will be achieved with a lower amount of active agent. This will increase the safety of the treatment by providing a significant increase in the therapeutic effect/side effect ratio. Hence, passive targeting of destructive drugs used in cancer treatment with PEG is a very useful approach. In addition, hiding foreign protein structures from the reticuloendothelial system with PEG is among the factors that pave the way for novel biotechnological drugs. Studies on the search for new ligands and the development of nonchromatographic affinity strategies for PEG- modified proteins will proliferate. Also, in the future, new PEGylated agents will increase the demand for stable and long-lasting drugs.
References
- Webster R, Elliott V, Park BK, Walker D, Hankin M, et al. (2009) PEG and PEG conjugates toxicity: towards an understanding of the toxicity of PEG and its relevance to PEGylated biologicals. PEGylated Protein Drugs: Basic Science and Clinical Applications pp. 127-146.
- Brannon PL, Blanchette JO (2004) Nanoparticle and targeted systems for cancer therapy. Adv Drug Deliv Rev 56(11): 1649-1659.
- Harvey RJ, Lund VJ (2007) Biofilms and chronic rhinosinusitis: systematic review of evidence, current concepts and directions for research. Rhinology 45(1): 3-13.
- Bhatia S (2016) Natural polymers vs synthetic polymer. Natural Polymer Drug Delivery Systems pp. 95-118.
- Duncan R, Vicent MJ (2010) Do HPMA copolymer conjugates have a future as clinically useful nanomedicines? A critical overview of current status and future opportunities. Advanced Drug Delivery Reviews 62(2): 272-282.
- Duncan R, Veronese FM (2009) Preface PEGylated protein conjugates: A new class of therapeutics for the 21st PEGylated Protein Drugs: Basic Science and Clinical Applications pp. 1-9.
- Benson HA, Roberts MS, Williams A, Liang X (Eds.) (2021) Fundamentals of drug delivery. Wiley, USA.
- D souza AA, Shegokar R (2016) Polyethylene glycol (PEG): A versatile polymer for pharmaceutical applications. Expert Opin Drug Deliv 13(9): 1257-1275.
- Veronese FM, Pasut G (2005) PEGylation, successful approach to drug delivery. Drug Discov Today 10(21): 1451-1458.
- Alconcel SN, Baas AS, Maynard HD (2011) FDA-approved poly (ethylene glycol)–protein conjugate drugs. Polymer Chemistry 2(7): 1442-1448.
- Gupta V, Bhavanasi S, Quadir M, Singh K, Ghosh G, et al. (2019) Protein PEGylation for cancer therapy: bench to bedside. J Cell Commun Signal 13(3): 319-330.
- Greenwald RB (2001) PEG drugs: an overview. J Control Release 74(1-3): 159-171.
- Asfour FH (2004) Molecularly reinforced polymers and self-assembled nanocomposites for secondary lithium batteries. Michigan State University, USA.
- Jang HJ, Shin CY, Kim KB (2015) Safety evaluation of polyethyleneglycol (PEG) compounds for cosmetic use. Toxicol Res 31(2): 105-136.
- Brady J, Dürig T, Lee PI, Li JX (2017) Polymer properties and characterization. Developing Solid Oral Dosage Forms, Academic Press, USA, pp. 181-223.
- Harris JM (1985) Laboratory synthesis of polyethylene glycol derivatives. Journal of Macromolecular Science-Reviews in Macromolecular Chemistry and Physics 25(3): 325-373.
- Kumbar S, Laurencin C, Deng M (Eds.) (2014) Natural and synthetic biomedical polymers. Newnes, United Kingdom.
- Tan DK (2021) Simple and low-cost manufacturing of customisable drug delivery devices and flexible sensors for biomedical applications. Doctoral dissertation, University of Sussex, England.
- Kannapiran S, Vitta S, Ranganathan B, Rajagopal V, Gimbun J, et al. (2022) PEG-PLA nanoformulation for breast cancer therapy. Trends in Biomaterials & Artificial Organs 36(S1): 76-82.
- Fu Y, Ding Y, Zhang L, Zhang Y, Liu J, et al. (2021) Polyethylene glycol (PEG)-related controllable and sustainable antidiabetic drug delivery systems. European Journal of Medicinal Chemistry 217: 113372.
- Sharifianjazi F, Irani M, Esmaeilkhanian A, Bazli L, Asl MS, et al. (2021) Polymer incorporated magnetic nanoparticles: Applications for magnetoresponsive targeted drug delivery. Materials Science and Engineering: B 272: 115358.
- Ghauri ZH, Islam A, Qadir MA, Ghaffar A, Gull N, et al. (2022) Novel pH-responsive chitosan/sodium alginate/PEG based hydrogelsfor release of sodium ceftriaxone. Materials Chemistry and Physics 277: 125456.
- Pervaiz F, Tanveer W, Shoukat H, Rehman S (2022) Formulation and evaluation of polyethylene glycol/Xanthan gum-co-poly (Acrylic acid) interpenetrating network for controlled release of venlafaxine. Polymer Bulletin pp. 1-25.
- Iovene A, Zhao Y, Wang S, Amoako K (2021) Bioactive polymeric materials for the advancement of regenerative medicine. Journal of Functional Biomaterials 12(1): 14.
- Dethe MR, Prabakaran A, Ahmed H, Agrawal M, Roy U, et al. (2022) PCL-PEG copolymer based injectable thermosensitive hydrogels. Journal of Controlled Release 343: 217-236.
- Lin CC, Anseth KS (2009) PEG hydrogels for the controlled release of biomolecules in regenerative medicine. Pharm Res 26(3): 631-643.
- Tourné-Péteilh C, Barège M, Lions M, Martinez J, Devoisselle JM, et al. (2021) Encapsulation of BSA in hybrid PEG hydrogels: stability and controlled release. RSC Advances 11(49): 30887-30897.
- Ziegler CE, Graf M, Beck S, Goepferich AM (2021) A novel anhydrous preparation of PEG hydrogels enables high drug loading with biologics for controlled release applications. European Polymer Journal 147: 110286.
- Fu Y, Ding Y, Zhang L, Zhang Y, Liu J, et al. (2021) Poly ethylene glycol (PEG)-related controllable and sustainable antidiabetic drug delivery systems. Eur J Med Chem 217: 113372.
- Yadav D, Dewangan HK (2021) PEGYLATION: an important approach for novel drug delivery system. Journal of Biomaterials Science, Polymer Edition 32(2): 266-280.
- Veronese FM (Ed.), (2009) PEGylated protein drugs: basic science and clinical applications. Springer Science & Business Media, Germany.
- Tong X, Lee S, Bararpour L, Yang F (2015) Long‐term controlled protein release from poly (ethylene glycol) hydrogels by modulating mesh size and degradation. Macromolecular Bioscience 15(12): 1679-1686.
- Kolate A, Baradia D, Patil S, Vhora I, Kore G, et al. (2014) PEG—a versatile conjugating ligand for drugs and drug delivery systems. Journal of Controlled Release 192: 67-81.
- Li R, Chen Z, Dai Z, Yu Y (2021) Nanotechnology assisted photo-and sonodynamic therapy for overcoming drug resistance. Cancer Biol Med 18(2): 388-400.
- Sylvestre M, Lv S, Yang LF, Luera N, Peeler DJ, et al. (2021) Replacement of L-amino acid peptides with D-amino acid peptides mitigates anti-PEG antibody generation against polymer-peptide conjugates in mice. J Control Release 331: 142-153.
- Zhong D, Wang Z, Zhou J, Wang Y (2021) Additive-free preparation of hemodialysis membranes from block copolymers of polysulfone and polyethylene glycol. Journal of Membrane Science 618: 118690.
- Grundler J, Shin K, Suh HW, Zhong M, Saltzman WM (2021) Surface topography of polyethylene glycol shell nanoparticles formed from bottlebrush block copolymers controls interactions with proteins and cells. ACS Nano 15(10): 16118- 16129.
- Leng C, Hung HC, Sun S, Wang D, Li Y, et al. (2015) Probing the surface hydration of nonfouling zwitterionic and PEG materials in contact with proteins. ACS Appl Mater Interfaces 7(30): 16881-16888.
- Owens III DE, Peppas NA (2006) Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. International Journal of Pharmaceutics 307(1): 93-102.
- Moghimi SM, Hunter AC, Murray JC (2001) Long-circulating and target-specific nanoparticles: theory to practice. Pharmacol Rev 53(2): 283-318.
- Li H, Wang Y, Tang Q, Yin D, Tang C, et al. (2021) The protein corona and its effects on nanoparticle-based drug delivery systems. Acta Biomaterialia 129: 57-72.
- Elsewedy HS, Al Dhubiab BE, Mahdy MA, Elnahas HM (2021) A review article on the basic concepts of drug delivery systems as targeting agents. Int J Pharma Med Biol Sci 10: 23-29.
- Harris JM, Chess RB (2003) Effect of pegylation on pharmaceuticals. Nat Rev Drug Discov 2(3): 214-221.
- Park H, Otte A, Park K (2022) Evolution of drug delivery systems: From 1950 to 2020 and beyond. Journal of Controlled Release 342: 53-65.
- de la Torre BG, Albericio F (2022) The Pharmaceutical industry in 2021. An analysis of FDA drug approvals from the perspective of molecules. Molecules 27(3): 1075.
© 2022 Yenilmez E. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






