- Submissions

Full Text
Polymer Science: Peer Review Journal
Investigation of Alcohols as Insulating Paper Aging Indicator for Power Transformers
Zhang E, Zheng H and Zhang C*
School of Electrical Engineering, Guangxi University, China
*Corresponding author: Chaohai Zhang, School of Electrical Engineering, Guangxi University, Nanning, China
Submission: January 14, 2022;Published: March 18, 2022

ISSN: 2770-6613 Volume3 Issue2
Abstract
There are still many defects in characterizing transformer insulation aging with furfural content in oil. For example, the field measurement value and detection accuracy are generally low, and the accuracy of evaluating the aging state is not enough. To overcome this shortage, the methanol (MeOH), ethanol (EtOH) and other low molecular weight alcohols are theoretically analyzed and experimentally verified as new features of aging evaluation for insulating paper in the transformer. Thus, the main reaction and formation path of cellulose degradation to low molecular alcohols were analyzed in this work at the atomic level. With the goal of providing references for precisely estimating the paper insulation state of field transformers.
Keywords: Transformer; Insulating paper; Aging assessment; Low molecular weight alcohols
Abbreviations: DP: Degree of Polymerization; DGA: Dissolved Gas Analysis; GC-MS: Gas Chromatography- Mass Spectrometry
Introduction
Oil-immersed transformers are mostly used in power supply and distribution systems. The insulation system within the transformer is mostly formed of insulating oil and paper, and its degree of aging dictates the transformer’s operating life [1]. Oil filters have been shown to increase the insulating oil’s performance [2], however the insulating paper is not changeable. So, the aging of the insulating paper is the critical factor that determines the insulation life of the transformer. In the aging process, chain scission of cellulose caused a decrease in the Degree of Polymerization (DP) and insulating paper’s mechanical properties, and even the insulating paper embrittlement occurred in severe cases; so, one function of DP is considered to reflect the aging of insulating paper directly [3]. However, according to the existing technology, the use of DP assessment is straightforward, but it is very difficult to obtain a pattern of key parts. In this case, the implication of this method to evaluate the aging degree of the insulation paper in the transformer is often unsatisfied in industry. At present, some scholars have used the Dissolved Gas Analysis (DGA) [4] as a feature of aging assessment to characterize the insulation aging status and fault types of transformers, which have had many results. However, this method is not a much strong relationship between the aging degree of the insulating paper and the feature gases in the oil, especially the low-molecular hydrocarbons mainly originated from the cracking of the insulating oil. Besides, Furfural dissolved in oil [5] (which is created only during the aging of insulating paper and is extremely stable) is widely regarded as a trustworthy measure for determining the insulation state of insulating paper. Regrettably, the content of furfural detected in field transformers during the early stage of aging is quite low. Therefore, it is difficult to meet the needs of industry by relying solely on furfural content to diagnose the insulation aging of transformers. Some effective new features are still needed to characterize the degree of insulation paper aging.
Recent studies results showed the presence of ethanol and methanol during the ageing of oil-impregnated cellulosic insulation materials which could be identified as oil-soluble markers [6]. With the development of Gas Chromatography-Mass Spectrometry (GC-MS), the relationship between the breakage of 1,4-β-glycoside bond in cellulose and the detection in paper-oil system during aging has been studied in recent years. Laboratory tests showed that methanol is mainly produced during the insulation aging of oil-impregnated paper at 60 ℃-120 ℃, regardless of whether the specimens are thermally upgraded. This means that contrary to the 2-FAL, the methanol is instantaneously produced after the opening of the 1,4-ß-glycosidic bonds, so it can be detected early in the oil. This finding provides a new idea for the study of new indicator of insulating paper’s aging assessment. At the same time, verification results indicated that thermal aging of LGA (levoglucosan) in oil produced a large amount of ethanol compared to methanol no matter what the conditions is [7]. Further investigates have shown that cellulose degrades more ethanol at higher temperature [8]. It can be assumed that ethanol is a by-product of the degradation of the intermediate product LGA, which could indicate the presence of hot spots. The limitation of alcohols as indicators is that they will be consumed by organic acids in the middle and later stages of aging, resulting in a decrease in content, which cannot correctly evaluate the aging state of paper insulation. Recently, our research group [9] has carried out related research on this issue and found the main organic acid species that consume alcohol. It lays a theoretical foundation for low molecular alcohols to predict the life of paper insulation.
Degradation of Cleavage Mechanism of Cellobiose
The main component of oil-immersed transformer insulating paper is cellulose that accounting for more than 90% of the insulating paperboard composition. Besides, there are about 6% hemicellulose and about 4% lignin. The basic composition of cellulose is cellobiose, which is combined by two molecules of β-D-glucopyranose linked by glycosidic bonds. Figure 1a characterizes the basic repeating unit constituting cellulose, and an amorphous unit cell model containing 30 cellobiose molecules is shown in Figure 1b. Each long chain of cellulose molecules is formed by the connection of many glucopyranose units, while the microscopic process of insulating paper aging is the process of breakage of cellulose macromolecules. The ReaxFF reaction force field is applied to the molecular reaction dynamics simulations of the high-temperature thermal degradation of cellulose, making it possible to explore the degradation of cellobiose at the atomic level. The amorphous cell model of cellulose was studied by using ReaxFF reaction field (the simulated cell density was 1.2g/cm3 after optimization, which is consistent with the actual density of electrical insulating paper used in engineering applications of 1.00~1.30g/ cm3). The reaction process of two pyran cyclic glycoside bonds cleavage and the opening of pyran rings during pyrolysis is shown in Figures 2a-2d. In addition, the model was simulated and analyzed, the evolution curve of the pyrolysis products with simulation time were obtained as shown in Figure 2e (the main products are H2O, glycolic aldehyde CH2OHCHO (C2H4O2), formic acid HCOOH (CH2O2), free radical CHO2, 1,2-dihydroxy ethylene CHOH=CHOH(C2H4O3), etc.) It is found that the initial disruption position of cellulose cellobiose in the initial cleavage phase mainly concentrated at O atoms, C5’-O5’ (C5-O5) and C1-O5 (C1’-O5’) of the glycosidic bond. Also, there are also minor dislocations at C1-C2 (or C1’-C2’) and C2-C3 (or C2’-C3’) sites.
Figure 1: Atomic Labeling Diagram of Cellobiose Molecule (C12H22O11) and Constructed Amorphous Cell Model.
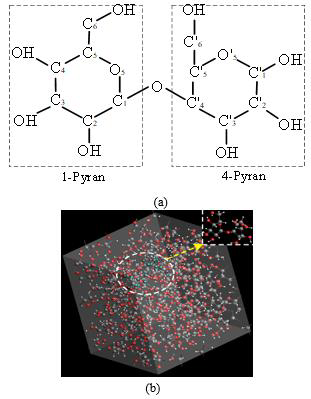
Figure 2: The reaction process of pyran cyclic glycoside bonds cleavage and pyran ring opening.
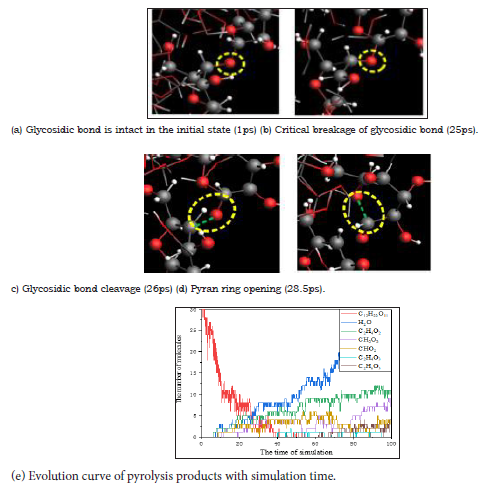
In the cleavage process, hydroxyl and hydrogen atoms in cellobiose may also have a finite fracture. With the progress of pyrolysis, the number of cellobiose (C12H22O11) molecule decreased sharply, while the number of water molecule increased rapidly. It was confirmed by FTIR analysis that the secondary and primary hydroxyl groups of the cellulose glucosyl ring were important factors affecting its chemical properties and physical strength, so (Figures 3a-3d) shows the simulation of the microscopic process of hydrogen dehydration of secondary hydroxyl groups by ReaxFF reaction molecular dynamics.
Figure 3: The reaction process of hydrogen dehydration of secondary hydroxyl groups.
Production Mechanism of Low Molecular Alcohol Compounds
This section infers how the low molecular weight alcohols are appearing in the transformers based on the statistical analysis of the fracture position of the initial bond of cellobiose in 2.1 and the simulation results of ReaxFF reaction force field.
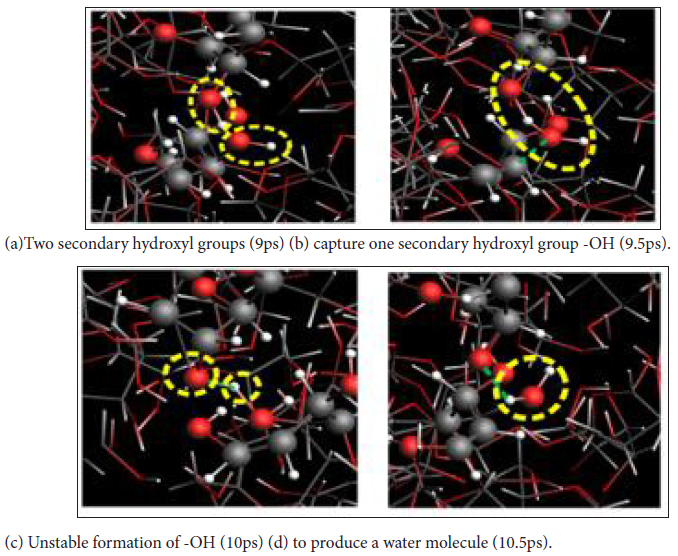
Production pathway of methanol
a. When the C5-C6 (or C5’-C6’) bond of the cellobiose is cleaving, the C6 (or C6’) and the hydroxyl group which are attached on C6 atom form methanol is shown in Figure 4.
b. When C1’-O5’ is cleaved, a C1’-O bond forms C1’-OH. If C1’-C2’ is also cleaved, the C1’ atom and hydroxyl group which are attached on C1’ atom form methanol. It is showed in Figure 4.
c. Figure 4 illustrates when the bonds of C1-C2 (or C1’-C2’) and C2- C3 (or C2’-C3’) are simultaneously cleaved, the C2 atom and its attached hydroxyl group form methanol.
Figure 4: Pathways for generation methanol indicator.
Production pathway of ethanol
a. The ReaxFF reaction force field was used to simulate the established cellulosic amorphous cell model. The formation of ethanol was found in the pyrolysis simulation products of the intermediate products is shown in Figure 5.
Figure 5: Formation of ethanol molecules.
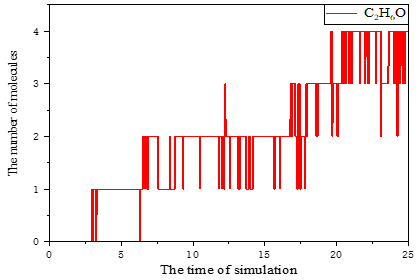
b. Using reaction molecular dynamics and phase analysis (pyrolysis experiments) to obtain cellobiose as a cellulose model compound, two major chemical reaction pathways such as rapid pyrolysis of cellulose to LGA and further degradation of LGA to ethanol were achieved. It is showed in Figure 6.
Figure 6: Schematic diagram of the main reaction pathway for the formation of LGA and ethanol by the cleavage of cellobiose.
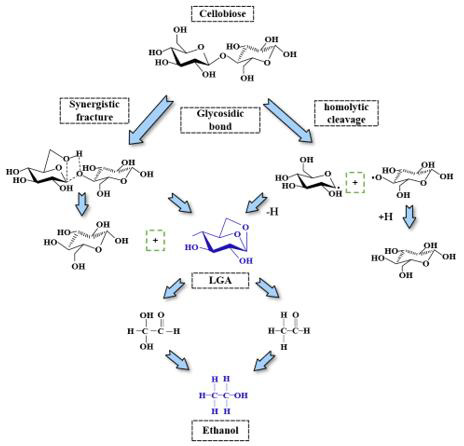
c. When the free hydroxyl group on C2 (or C2’) on cellobiose is cleaved, and the C1-O5 (or C1’-O5’) bond cleaves with the C2-C3 (or C2’-C3’), the newly formed hydroxyl groups on C1-C2 and C1 form ethanol.
d. Reference points out that the main product of cellobiose cleavage contained glycolaldehyde (CH2OHCHO). Some of the stainless-steel materials used inside transformers contain nickel as a good catalyst for hydrogen storage reactions. Meanwhile, the cracking of insulating oil can provide hydrogen (H2). Therefore, the reaction of glycolic aldehyde under the catalysis of nickel molecules will produce ethylene glycol, and the chemical equation is shown below:
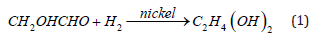
In this paper, a detection system for aging characteristic products of insulating paper dissolved in oil based on GC-MS was designed, and the stability test and analysis of several cellulose degradation products such as methanol, ethanol and n-butanol were carried out under different aging temperatures (60 ℃, 90 ℃, 110 ℃ and 120 ℃. The results confirmed that methanol and ethanol have the best stability and are suitable for the ageing characteristic products of transformer paper insulation. But methanol has low relative molecular mass and low boiling point. In the actual experimental test, methanol is volatile, so it highly demands on the testing equipment and environmental conditions. Relatively, by the detection and analysis of the dissolved aging products in 110~220kV transformer oils of more than running 30 sets in the grid, the results show that the detectable concentration of ethanol in oil is higher than that of methanol and furfural. Therefore, ethanol can be treated as new features of chemical detection of insulating paper degradation of oil-immersed power transformers.
Conclusion
Considering the shortcomings of the traditional insulation aging indicator of filed transformer in characterizing the aging state of insulating paper, in this paper, the summary of the applicability for low molecular weight alcohols is conducted. By analyzing the aging decomposition mechanism and the chemical bond rupture situation of insulating paper cellulose, the principles of generating and formatting way of methanol and ethanol has been investigated.
References
- Christina A, Salam M, Rahman Q, Wen F, Ang SP, et al. (2018) Causes of transformer failures and diagnostic methods – A review. Renew Sust Energ Rev 82(1): 1442-1456.
- Li S, Ma H, Saha T, Yan Y, Guangning W (2018) On particle filtering for power transformer remaining useful life estimation. IEEE Trans Power Del 33(6): 2643-2653.
- Zhang E, Zheng H, Zhang C, Wang J, Shi K, et al. (2021) Aging state assessment of transformer cellulosic paper insulation using multivariate chemical indicators. Cellulose 28(4): 2445-2460.
- Zheng H, Zhang Y, Liu J, Wei H, Zhao J, et al. (2018) A novel model based on wavelet LS-SVM integrated improved PSO algorithm for forecasting of dissolved gas contents in power transformers. Electr Power Syst Res 155: 196-205.
- Lin Y, Wei C, Tao F, Li J (2019) Aging assessment of oil-paper insulation of power equipment with furfural analysis based on furfural generation and partitioning. IEEE Trans Power Del 34(4): 1626-1633.
- Jalbert J, Gilbert R, Tetreault P, Morin B, Deziel DL (2007) Identification of a chemical indicator of the rupture of 1,4-β-glycosidic bonds of cellulose in an oil-impregnated insulating paper system. Cellulose 14(4): 295-309.
- Jalbert J, Rodriguez-CE, Duchesne S, Morin B, Ryadi M, et al. (2015) Kinetics of the production of chain-end groups and methanol from the depolymerization of cellulose during the ageing of paper/oil systems. Part 3: extension of the study under temperature conditions over 120 °C. Cellulose 22(1): 829-848.
- Rodriguez-CE, Duchesne S, Jalbert J, Ryadi M (2015) Understanding ethanol versus methanol formation from insulating paper in power transformers. Cellulose 22(5): 3225-3236.
- Zhang E, Liu J, Fan X, Zhang Y, Zhang C (2021) Reduction mechanism of alcohols contents caused by acids during oil-paper insulation aging. IEEE Trans Dielectr Electr Insul 28(6): 1867-1874.
© 2022 Chaohai Zhang. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






