- Submissions

Full Text
Polymer Science: Peer Review Journal
Impact of Resin Treatment on the Crystallinity of Finished Cotton
Nasr Litim*, Ayda Baffoun and Saber Ben Abdessalem
Textile Materials and Processes Research Unit MPTex, National Engineering School of Monastir University of Monastir, Tunisia
*Corresponding author: Nasr Litim, Textile Materials and Processes Research Unit MPTex, National Engineering School of Monastir University of Monastir, Monastir 5019, Tunisia
Submission: February 12, 2022;Published: February 24, 2022

ISSN: 2770-6613 Volume3 Issue2
Abstract
This paper presents an investigation of modified glyoxalic (DMDHEU) and co-polymer acrylic resins effect on the crystallinity and crystallite Size of finished cotton. Using a modern technical analysis, ATRFTIR and X- ray diffraction, is very significant to describe structural modification of cellulose fiber treated with two types of resins. Investigation of the effect of used resins at various finishing conditions (curing temperature, curing time, drying temperature, drying time and resins concentration) on crystallinity and crystallite size of finished cotton can explain the phenomena encountered during this kind of treatment. The obtained results show the pronounced influence of treatment conditions (higher curing) that reduce the crystallinity and crystallite size of treated cotton.
Keywords: Resins; Finishing; Crystallinity; Cotton
Introduction
Crosslinkers are generally small molecules that enclose a number of functional groups (commercially known as resin). They are able to link with a number of dynamic groups in the polymer such as hydroxyl groups in cellulose. In order to develop the durable press applied on cotton fabric, the advanced application has been to link a covalently crosslinking agent with adjoining cellulose chains inside fibers [1-3]. Previously, we have studied the effect of two famous finishing resins; acrylic and Glyoxal resins applied according to various finishing conditions, on the mechanical and surface properties of different cotton denim fabrics. On the other hand, we have detail and explain the effect of yarn composition and starch sizing composition on the quality of resin treatment. The effect of resins treatment conditions: resin concentration, curing temperature, curing time, dry time and dry temperature. Obtained results showed that sized cotton yarn is most preserving the mechanical properties under curing conditions; unlike stretch cotton yarn is more sensitive to high curing temperature and time [4,5]. The aim of this paper is explaining the impact of Glyoxalic and acrylic resins on the crystallinity and crystallite size of finished cotton yarns. After finishing treatment, the structure and organization of cotton cellulose was studied using X-ray diffraction and ATR FTIR spectroscopy. It is known that cellulose microfibrils in cotton are composed of crystalline and amorphous regions. Crystallinity index is known to measure the relative amounts of amorphous and crystalline cellulose. The obtained change in the amorphous and crystalline of cellulose fiber is a result of intermolecular and intramolecular hydrogen link [6]. Today, a characterization of X-ray diffraction allows describing the changes of crystallinity values in the treated cotton yarns and understanding the resins’ treatment concept. We found that the change in the internal structure of the cotton is related to a chemical characteristic of resins and to curing conditions (temperature and time).
Experimental
The experiment consisted in using a 100 (%) cotton yarns Nm 14. It is the most used cotton as warp and weft in the denim woven structure. Two types of finishing resins are used. Table 1 gives details of used resins and catalysts. These resins are usually used in the permanent press and wash and wear treatments. The selected resins are usually used to improve the durable press properties of fabrics. In addition, modified Glyoxalic and acrylic resins can make up ester links with the cellulose chains [7] as shown in (Figures 1a & 1b). For the resin treatment, we used a pad dry cure process. The yarn is impregnated in a bath containing a diluted resins solution: resins agent (resin), catalyst (15(%) of resins weight) and water. Drying is carried out in a ream “Mathis AG-Switzerland”, while the curing step is realized in a drying oven. To examine sequentially the comparative effect of resins on the mechanical’s properties of finished cotton yarns. Details of experiments conditions shown in Table 2. ATR FT-IR Spectroscopy “PerkinElmer-France” used in the investigation of treated yarns. To determine the crystallinity at different conditions, the treated samples were discrete on a stump placed in the room of a (D max) shaft Analytical X-Ray diffractometer “X’pert Pro MRDNetherlands” with a wavelength of 1,54 (Å). The generator intensity was 40 (kV) and generator current was 50 (mA). The samples were then scanned using (2ϴ=5-100°). The rotation speed of clinometers was 8°/min. The crystallinity index CI ((%)) is defined in the following equation (1):
CI=100 [(I 002 – I am)/I 002] Eq. (1)
Figure 1: a) Cross-linking reaction of a cellulose with the modified Glyoxalic resins (DMDHEU) in presence of catalyst, curing temperature is among 140-160 (°C), curing time 5 (min). b) Cross-linking reaction of cellulose unit with co-polymer acrylic at 95-140 (°C) curing time 5 (min) and for pH=7.
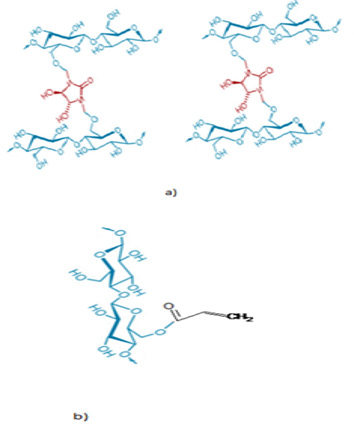
Table 1: Basic properties of resins.
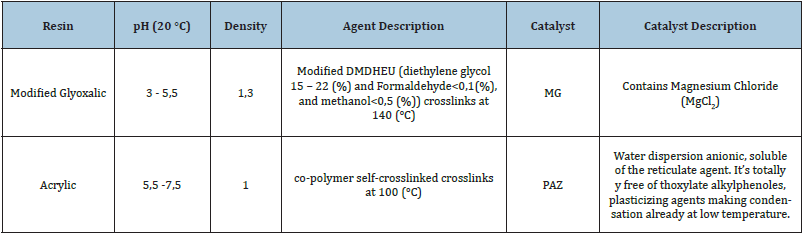
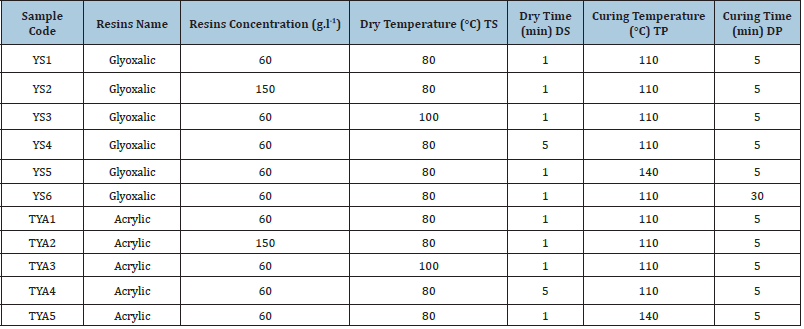
Table 2: Details of experiments conditions.
Where (I 002) presents the crystalline peak of the highest intensity at 2ϴ (between 22° and 23°), and ‘I am’ is the lowest intensity at 2ϴ (between 18° and 19°), generally used for cellulose I [8]. The crystallites sizes were definite with a diffraction model resulted from the (L002) plan of treated cotton yarns. In order to calculate the crystallite size with (L 002) plan [9], we used a famous Sherrer’s equation (2) as shown below:
L 002= Kλ/ (β cosϴ) Eq. (2)
Where λ is the wavelength of radiation (1,54Å), ϴ is the celebrated “Bragg” angle of the diffraction peak in degree, and K is the constant habitually taken as 0, 9 and is the full width at half maximum intensity in radians measured at 2ϴ.
Results and discussion
Characterization of Resins and treated cotton with ATR-FTIR spectroscopy
Figure 2: ATR-FTIR spectra of modified Glyoxalic and acrylic resins (wave-number from 400 to 4000 (cm-1)).
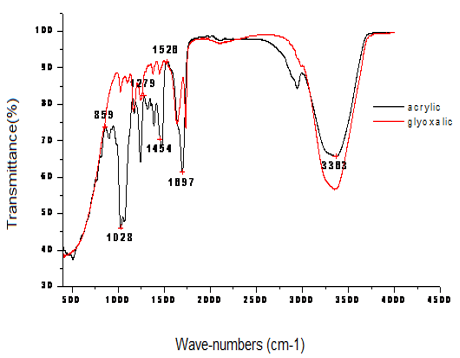
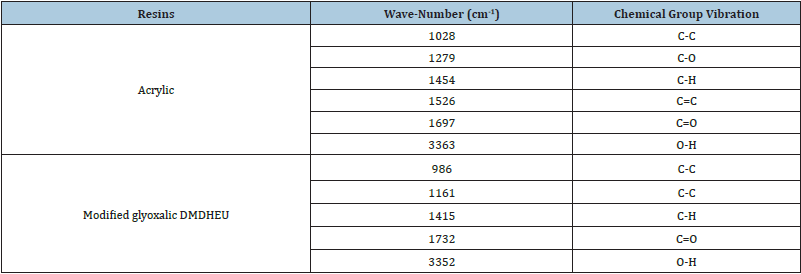
In order to provide a further clarification of the effect of crosslinking agent reaction with a natural fiber, we may begin by studying the functional chemical group. Actually, ATR-FTIR is yet an extra advanced technique to investigate hydrogen link in cellulose. Since 1950, The FTIR has been the best means to examine the full area of hydroxyl group stretching in the ATR-FTIR wavenumber of cellulose I (cotton) [10]. Figure 2 shows the ATR- FTIR spectra of modified Glyoxalic and acrylic resins. It is clear that the detected values of wavenumbers correspond to a chemical group vibration for the studied finishing agent. The modified Glyoxalic DMDHEU has a specific wavenumber 3352 (cm-1) that corresponds to the specific O-H group. As for acrylic agent, it has a wavenumber 1697cm-1 that corresponds to specific C=O group. Thanks to ATR-FTIR, the wavenumbers corresponding to the chemical group vibration of resins are presented in Table 3. The ATR-FTIR spectra of untreated and treated cotton yarns with Glyoxalic and Acrylic resins at different finishing conditions and results is shown in Figure 3.
Figure 3: ATR-FTIR spectra of untreated and treated cotton yarns with glyoxalic and acrylic resins at different finishing conditions (wave number from 400 to 4000 (cm-1)).
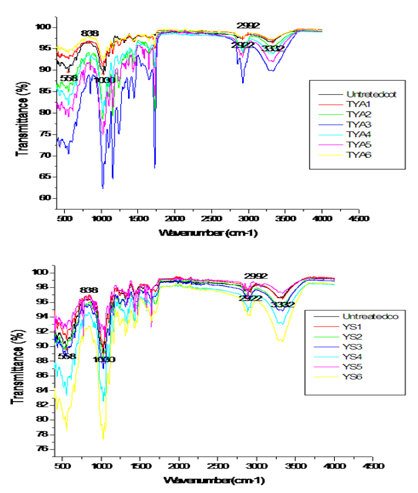
Table 3: Corresponding wavenumbers to chemical group vibration of two resins.
It has long been known that cotton has an important number of OH groups. The large peak above 3300 (cm–1) is designated to the OH group and the low group at 1640 (cm–1) are owed to hydration. After the resin treatment, new peaks occur at 1730 (cm–1), which can be attributed to C-O stretching vibration of groups in the resin. These peaks show that the resin agent has been successfully applied to the surface of cotton. They also have a chemical reaction that was taken to create the ether bonds in OH group of modified Glyoxalic (DMDHEU) with cellulose fiber [7,11]. In fact, many factors can be the origin of change in the wave-number peaks obtained. They have changed as a function of resins concentration, dry temperature and time, curing temperature and time. In our case, the change of the obtained wave-number peaks could not be in relation with a catalyst nature. Yang et al. [12] note that the quantity of the catalyst used in a reaction is related to the reactivity of the reactants and the curing temperature and curing time. Catalyst categories, the quantity of catalyst, or the curing temperature and curing time are the most factors that can be varied to help them and make better their reactivity. It is known that excessive curing temperature and curing time can burn cellulosic fabrics and color them to yellow. A temperature of 180 (°C) is usually used as the curing temperature for formaldehyde-free resin in durable press treatment [12]. Using ATR-FTIR, the researcher approves the crosslinking reaction between resins and fibers after finishing.
Characterization of treated cotton yarns with X-ray diffraction
In order to explain the specific effect of used resins and applied curing and drying conditions on mechanical properties, crystallinity and crystallite size of cotton, yarns are treated with Glyoxalic and acrylic resins at different finishing conditions. We summarize the obtained results of the X-ray diffraction analysis, in Table 4. Referring to Table 4, this shows the estimated crystallinity index CI (%) and crystallite size of untreated cotton and treated with Glyoxalic and acrylic resins. It is clear that the value of the estimated crystallinity index CI (%) for all treated samples is less than CI (%) of untreated cotton. We deduce that the crosslinking reaction affects the crystallinity of the cotton whatever the type of resin used, or the treatment factors (concentration, temperature and time) applied. Nevertheless, the crystallite size values of the treated samples vary depending on the nature of the resin. On the other hand, it is clear that the concentration of the resin and the polymerization temperature have caused a visible change in the size of the crystallites. Moreover, Ericka et al., suggest that increasing the concentration of DMDHEU affects the hydrogen bonding zones between the cellulose molecules. When the intermolecular crosslinking commences, the competition between the (-OH) groups of cellulose and the glycol will take place. In fact, high temperatures as well as the fragility of cellulose (-OH) groups are factors that could have influenced the non-linear extension of crystal size. From Table 4 we note that the crystallite sizes of the yarns treated with the two Glyoxalic and acrylic resins are lower than that of untreated cotton (3.94nm). Moreover, Weilin Xu [10] observed a reduction in the crystallinity of the cotton cross-linked with the resin (BTCA: butane carboxylic acid). He mentioned that it is possible for the properties to be improved with each crosslinking agent applied to the crimped cotton fiber, with different effects on the cotton structure. This is accompanied by changes in the degree of crystallinity of the cellulose in the fiber. In addition, he indicated that crystallinity is closely related to the size of fiber crystallites [6].
Table 4: The crystallinity index and the crystallite size of treated and untreated cotton yarns.
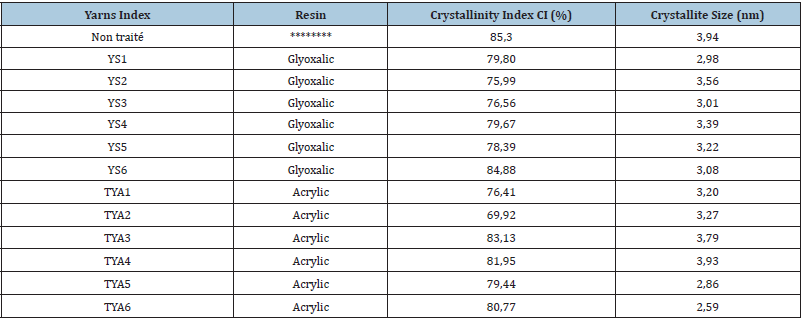
Subsequently, Parikh et al. [6] reported such a finding; a reduction in crystallinity in the cross-linked cotton treated with DMDHEU and citric acid [13]. In addition, our results show a reduction in crystallite sizes that can be related to changing drying conditions. Moreover, Hu & Hsieh [13] analyzed their cellulose samples treated with DRX. They showed that a decrease in crystallite size is typical throughout the drying step. Deformation could occur in the inter-crystalline and inter-fibril regions and could be almost on the surface of the crystals (crystalline zone) when the intermolecular bond is created, which causes the crystallite size to be reduced without substantially affecting the total crystallinity dry fibers [13]. Figure 4 presents X-Ray diffraction of samples treated with modified Glyoxalic resins and acrylic resins at various finishing conditions. A remarkable change was observed with intensity peaks for variation (2ϴ=5-100°). It is clear that the modification of finishing conditions (dry temperature, dry time, and curing temperature, curing time and resins concentration) has an important effect on the microstructure of cotton, especially for the (2ϴ=18°) and (2ϴ=22°). Referring to (Figure 4), it is clear that the X- ray specters of different conditions applied YSi1-6 and TYAj1-6 have an intensity that results from special comparison to one to another. This change is certainly due to the reaction of resins agent’s characteristics or to the finishing parameters (curing temperature and time, dry temperature and time). The constitution of cotton was customized with a head reaction with modified Glyoxalic (DMDHEU). The diffusion of resins molecules added to the microstructure of cotton was showed with the L002 plan. Referring to (Figure 4), the appearance of small peaks near values of (2ϴ=78°) and intensity of 100 especially for sample TYA1 is probably due to the finishing condition or catalyst reaction. We also can conclude from (Figure 4) that the appearance of a full peak with a higher intensity of 800 near values of (2ϴ=78°) for sample YS2 is probably due to higher resins agent concentration used equal 150g.l-1 that is superior to applied concentration of other samples. A modest peak also appears with an intensity of 300 near values (2ϴ=38°) for sample YS5 is probably due to a higher curing temperature of 140 (°C) compared to other treated samples. This X-ray result led us to point out that the effect of resins concentration and curing conditions is significant on the X-ray diffraction of various treated cotton yarns.
Figure 4: X-Ray diffraction of treated cotton yarns with modified Glyoxalic DMDHEU and Acrylic resins at various finishing conditions.

References
- Petersen H, Mark H, Wooding NS, Atlas SM (1971) Crosslinking chemicals and the chemical principles of the crosslinking finishing of cotton in chemical after treatment of textiles, Wiley-Inter-science, New York, USA.
- Welch CM, Andrews BA Kottes (1989) Ester crosslinks: A route to high performance non-formaldehyde finishing of cotton. Text Chem Color 21: 13-17.
- Wakelyn PJ, Bertoniere NR, French AD, Thibodeaux DP, Triplett BA, et al. (2006) Cotton fiber chemistry and technology. Taylor and Francis Group, Boca Raton, Florida, USA.
- Litim N, Baffoun A, Khoffi F, Hamdaoui M, Abdessalem SB, et al. (2017) Effect of finishing resins on mechanical and surface properties of cotton Denim fabrics. The Journal of The Textile Institute 108(11): 5.
- Litim N, Baffoun A, Hamdaoui M, Abdessalem SB (2017) Investigation of resin treatment effects on the mechanical, thermal and surface properties of cotton yarns. Journal Transylvanian review 15(13).
- Parikh DV, Thibodeaux DP, Condon B (2007) X-ray crystallinity of bleached and crosslinked cottons. Text Res J 77(8).
- Cooke TF, Weigmann HD (1982) The chemistry of formaldehyde release from durable press fabrics. Text Chem Color 14: 136-144.
- Alexander LE (1969) X-ray diffraction methods in polymer science. Wiley Inter-science, London, United Kingdom.
- Segal L, Creely JJ, Martin AE, Conrad CM (1959) An empirical method for estimating the degree of crystallinity of native cellulose using X-ray diffractometer. Text Res J 29: 786-794.
- Weilin X (2003) Effect of crosslinking treatment on the crystallinity, crystallite size, and strength of cotton fibers. Text Res J 73(5): 433-436.
- Hindeleh AM (1980) Crystallinity, crystalline size and physical properties of native Egyptian cotton. Text Res J 50: 667.
- Yang CQ, Wei W, Lickfield GC (2000) Mechanical Strength of durable Press finished cotton Fabric. Text Res J 70(2): 143-147.
- Hu XP, Hsieh YL (2001) Effects of dehydration on the crystalline structure and strength of developing cotton fibers. Textile Research Journal 71(3): 231-239.
© 2022 Nasr Litim. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






