- Submissions

Full Text
Polymer Science: Peer Review Journal
Thermal Stability of Hole Transport Material Organics: Carbazoles and Phenyl Benzidines
Velasco IA1, Luna JS2* and Morales JM1*
1Institute of Industries, Universidad del Mar, México
2Institute of Ecology, Universidad del Mar, México
*Corresponding author: Javier Salinas- Luna and Juan Mentado-Morales, Institute of Industries, Institute of Ecology, Universidad del Mar, Puerto Angel, C P 70902, San Pedro Pochutla Oaxaca, Mexico
Submission: January 19, 2022;Published: February 09, 2022

ISSN: 2770-6613 Volume3 Issue1
Abstract
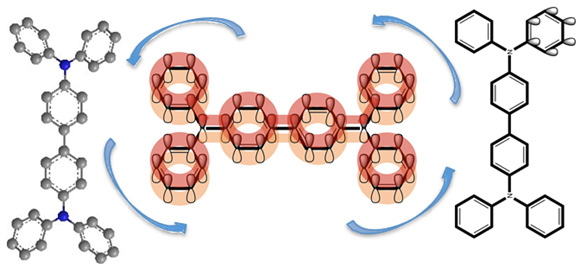
Compounds derived from carbazoles and phenyl benzidines have been used as organic dyes. In fact, due to their chemical structure and their conjugated bonds, they have aroused interest in being studied for their ability to transport electrons, which makes them good candidates to be used in solar cells, which have been called Dye Sensitized Solar Cells and Hole Transport Material. With the knowledge of the thermal properties obtained experimentally through thermal analysis, it is possible to establish the structureenergy relationship of carbazoles and phenylamines, as well as the thermal stability. Based on this, two carbazoles and two phenyl benzidines are feasible compounds with semiconductor capacity to be used in solar cells. The thermal results of the analyzed compounds show that the phenyl benzidine derivatives have a higher molar heat capacity, which is related to the resonance capacity of the electron density of the conjugated double bonds to generate electron density tubes in the in-phase structures solid (Figure 1). To understand the intramolecular movements of N,N’-Bis(3-methylphenyl)-N,N’-diphenyl benzidine and 1,3,5-Tris(diphenylamino)benzene, Raman spectra are presented.
Figure 1: Resonance capacity of the electron density of the conjugated double bonds.
Keywords: Heat capacities; Thermal stability; Raman spectra; Carbazoles; Phenylbenzidines
Introduction
Past, present and future human activities have a common factor, the use of “energy sources”. With this principle the human being has been able to exploit and manipulate the energy-providing resources that nature offers, such as non-renewable energies: coal, natural gas, oil and nuclear energy. Also, it has been possible to use renewable energy sources, such as geothermal, hydroelectric, tidal and biomass sources). However, the use of these sources leads to modification of natural habitats. Therefore, scientists and technologists are developing technology capable of harnessing a source of energy that has been available since long before the emergence of the human being, that being “solar energy”. The oil crisis, combined with global warming and acidification of the oceans from the emission of greenhouse gases, as well as from other gases harmful to the environment such as chlorofluorocarbons, has motivated part of the scientific community to develop, as technologists, technology capable of harnessing solar radiation and transforming it into electrical energy. Today these devices are involved in most of our activities and are known as “solar cells”. It was in 1954 at Bell Labs that the first silicon-based inorganic cell was developed with an efficiency of 6%. Subsequently, silicon was used in crystalline form, increasing efficiency to 24% [1].
Nowadays, the use of these devices is proliferating throughout different countries in Europe, Asia and America, and predominantly in the United States. Although these devices are a desirable option for their use of solar energy, a means of reducing dependence on non-renewable energy sources, they have three drawbacks: Firstly, current solar cells have a low efficiency rate of about 35%. Secondly, from an environmental perspective their manufacture is very problematic because producing the high-quality silicon from which they are constituted leaves a chemical footprint (a trace of chemical contamination). This is because the related purification process requires the use of large amounts of HCl and H2SO4 [2]. Thirdly, once the useful life of the solar cells expires, disposal of the materials comprising the dead cells becomes the problem because there is no program for the collection and even less prospect for the recovery of the heavy metals that these cells contain, such as Cd, Ga and Cr, among others, heavy metals that, if not recovered, will pass into the food chain and emerge as contaminants. Therefore, in response to these issues, this work investigates the usefulness of other types of compounds that would reduce or suppress the use of conventional materials in the manufacture of solar cells, which currently relies on the use of silicon and organometallic substances.
Research Background
Significantly, in 1960 it was discovered that several dyes, such as methylene blue, had photoconductive properties [3]. A decade later, via the work of Tang and VanSlyke, research was conducted for the creation of cells containing organic compounds currently known as Organic Light Emitting Diodes (OLED) [4]. However, up to the present, studies have only considered a small number of OLEDs in solar cells. Therefore, to support the implementation of new OLEDs for the design and construction of solar cells that are more efficient than conventional solar cells, it is necessary to further the study of related energy and thermal parameters. Based on the studies conducted by our research group, the reports pertinent to the work are as follows:
i. Organic compounds derived from carbazoles and phenylamines make high- capacity semiconductors with high triple-energy [5]. This has prompted the synthesis of this type of compound, an example being the research carried out by Zhang et al. [6]. They synthesized semiconductor molecules with a central unit of 1,4-bis (carbazole) benzene: (Figure 2a). The synthesized compounds were thermally stable and presented glass transition temperatures of 141-157 ºC, values determined through the use of thermal analysis techniques such as Differential Scanning Calorimetry (DSC) and Thermogravimetric Analysis (TGA). In addition, they reported that properties such as stability and semiconductor capacitance in the compounds can be improved by increasing the number of diphenylamine groups [6]. Quynh et al. [7] studied the transport of electrons in compounds derived from 4-(9H-Carbazol) triphenylamines (Figure 2b) concluding that these compounds have adequate thermal and morphological stability. ii. Peter Strohriegl et al. [8] studied two types of compounds. The first consisted of biphenyls substituted with two carbazole, compounds that showed high energy (2.98-2.98eV) and glass transition. The second were bipolar carbazole, linked to a triazine unit responsible for converting the material into a semiconductor. The melting temperature, crystallization, glass transition and decomposition temperature of these compounds were determined via DSC and TGA [8].
iii. Costa et al. [9], using DSC and TGA, gathered data on heat capacity, purity and thermodynamic properties such as vapor pressure, enthalpy and sublimation entropy, including the enthalpy of 1,4-bis (diphenylamino) benzene (Figure 2c) and tetra-Nphenylbenzidine vaporization [9].
iv. El-ghamdour A et al. [10] investigated on the Optical and electrical properties of nanostructured organic thin films of N,N′- diphenyl-N,N′-di-p-tolylbenzene- 1,4-diamine and the possibility of using it as a hole transport layer in optoelectronic devices [10].
Figure 2: The structures of carbazole and phenyl amine benzene.

v. Mendoza-Ruiz et al. [11] reported the thermal and thermochemical capabilities of two OLED compounds, Tetra- N-phenylbenzidine and 4,4´-Bis (N-carbazolyl)-1, assessing 1´-biphenyl using DSC and semi-micro combustion calorimetry. They determined that the compound that best functions as a photoconductive structure is Tetra-N-phenylbenzidine since its molecular structure has greater thermal stability and greater heat capacity and forms the most stable compound based on its enthalpy value in solid and gas phase formation [11]. Recently, Mentado- Morales et al. [12] they published a theoretical-experimental work on the thermodynamic properties of two Hole Transport Materials (HTM) to Develop Solar Cells: 1,3-Bis (N- carbazolyl)benzene and 1,4-Bis(diphenylamino)benzene [12].
Methodology (DSC, NMR, Raman, CC and TGA)
The purity of the organic compounds is essential for the quantification of the thermal and thermochemical properties depend on the purity and potentiate the energy efficiency of a finilbenzidine and carbazole thin film, so it is essential that the samples have a high purity. Therefore, the first step in all experimental work in thermochemistry is to increase the purity of organic compounds using knowledge of the synthesis processes to identify the corresponding traces and separate them by recrystallization or chromatography methods in organic solvents to obtain a purity of at least 99.9%. Subsequently and in accordance with the experimental procedure, will characterize the structures of phenylbenzidines and carbazoles through the 1H and 13C spectra obtained by NMR and Raman spectra, respectively. With this characterization the chemical composition of each structure will be guaranteed. The differential scanning calorimetry DSC technique is used to determine the purity of substances, heat capacity, glass transition temperature, enthalpy and entropy of fusion. In the case of sublimation and vaporization enthalpies the Thermogravimetric Analysis (TGA) technique is used [11,12].
The heat capacity determination is through a DSC where the procedure consists of; Take a baseline, this was obtained by placing an empty sealed cell in the reference cell holder and an empty unsealed cell in the sample cell holder. After performing the baseline, a test experiment is performed with synthetic sapphire, it was placed in the empty unsealed cell and its Cp is determined to confirm that the baseline is adequate to carry out the experiments. Subsequently, a sample line is made, this is obtained by placing between 5 to 7 mg of the sample in the sample cell with a nitrogen flow of 50 mLmin-1 to ensure an inert atmosphere in all compounds. The heating rate and the temperature interval are always the same for each compound, adding an isotherm in order to stabilize the beginning and end of each experiment with a rate of heating of 2 °Cmin-1. After performing the two lines, the analysis is performed through the comparison method using these two lines to obtain the heat capacity of the compounds. For determination of the combustion energy and enthalpy of formation of the phenylbenzidines and carbazoles in the crystalline phase, the best technique so far is by calorimetry of macro-combustion (CC) and semi-micro combustion, by these techniques the energetic parameters are determined with high precision and with an error rate of up to 0.5%.
Discussion
Regarding to the previously cited studies, the following can be observed:
a. The experimental analysis of the thermal and thermochemical properties of these compounds are deficient.
b. In the studies developed by Mendoza-Ruiz et al. [11], we observed that the studied compounds, carbazole, contain less satisfactory thermochemical properties than phenylbenzidines because these last undergo a vitreous transition that indicates better prospects for use in solar cells. This is due to in the vitreous phase they do not maintain the rigidity of the solid phase and, where neither can be considered as liquids, and nothing is done to prevent their use in solar cell devices. The vitreous phase, from a chemical perspective, is a semi-solid phase that favors the overlapping of conjugated π bonds within the molecules and thus the occurrence of hole- transportation necessary for the transport of electrons. Additionally, in the works of Costa et al. [9] and Mendoza-Ruiz et al. [11], we can observe that phenylbenzidines have a greater heat capacity and thermal stability compared to other OLEDs structures and are, therefore, the structures that would function far better as thin films for solar cells.
c. In the previous studies, with the exception of the studies reported by Mendoza- Ruiz et al. [11], the compounds did not exhibit thermochemical properties, such as enthalpy of standard molar formation (DfH°) in solid and gaseous phases, much less the prospect of Gibbs energy (DfG°), which is a criterion of spontaneity and/or directionality, important for an understanding of the energy-structure relationship of the compounds.
d. In the organic energy-structure study is important to use a thermal analysis, to get their thermal stability and phase changes temperature, especially for changes related to glass transition and heat capacity, it is indicated for each compound. e. The Design and procurement of thin films based on OLEDs structures for topographic study is a very rare circumstance. Only one report about these was found but without a complete thermodynamic study to explain the energy- structure relationship.
The review of scientific reports conducted by the participants in this work prompts a question: why the thermochemical information about OLED structures is so limited and scarce when thermochemical properties are transcendental to a knowledge and understanding of the relationship between structures and energy. As a group study, we attribute the information about the thermochemical properties of this type of OLED structure to the difficulty involved in determining such via estimation methods or theoretical calculations, since, due to its size and structural arrangement, estimates can be very imprecise. In experimentation the most accurate and proven technique is calorimetry via static pump combustion. However, this technique is destructive and amounts of about 10 grams of substance are required to perform a complete calorimetric study, a situation which limits the possibility of thermochemical study.
Results
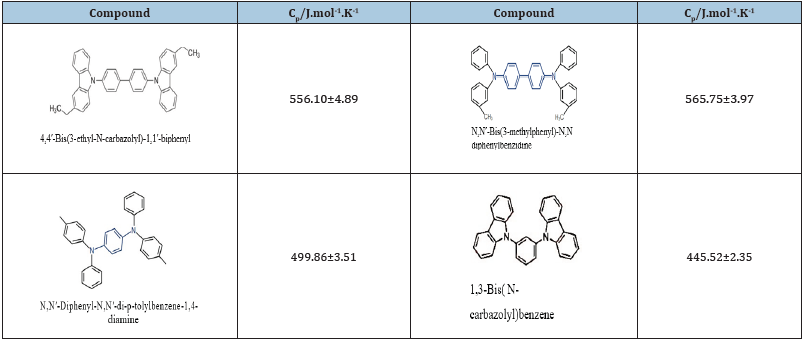
One of the important parameters to know the thermal stability of organic structures is their heat capacity, which is measured by DSC. Table 1 report the heat capacities of four compounds: 4,4′-Bis(3- ethyl-N-carbazolyl)-1,1′-biphenyl, N,N′-Bis(3-methylphenyl)-N,N′- diphenylbenzidine, N,N′-Diphenyl-N,N′-di-p-tolylbenzene-1,4- diamine and 1,3-Bis(N-carbazolyl)benzene [11]. In (Figure 3) The Raman spectra for two phenylbenzines are presented beside with the intramolecular movements of each compound. The spectra were obtained using a Bruker Avance III 500MHz and a fibercoupled EZRAMAN-N of Enwave Optronics, previously calibrated by obtaining the standard Raman spectrum for acetonitrile. Therefore, the obtained peaks number and the intensity in the signals of the Raman spectra coincide completely with the greater number of bond angles, torsion angles, and bond lengths presented in the N,N’-Bis(3-methylphenyl)-N,N’-diphenylbenzidine in comparation to the 1,3,5-Tris(diphenylamino)benzene, such as it is shown in the following (Figure 3).
Figure 3: Raman spectra of N,N’-Bis(3-methylphenyl)-N,N’-diphenylbenzidine and (a) 1,3,5-Tris (diphenylamino) benzene (b). The , and r represent bond angles, torsion angles, and bond lengths, respectively.

Table 1: Comparison of the heat capacities of four compounds.
Conclusion
The molecules thermal stability is defined based on their greater ability to absorb energy without decomposing or undergoing a phase change. The magnitude of Cp is strongly linked to the greater number of bonds, intra- and inter-molecular vibrational motions. Consequently, the molecules of the phenilbenzidines have higher Cp in a wide range of temperatures than the closed structures (carbazole), a parameter which is related to the resonance capacity of the electronic density of the conjugated double bonds to generate electronic density tubes in solid phase structures. This means that the derivatives of phenilbenzidines are outlined as suitable structures to be solar sensitizers in thin plates due to their greater thermal stability, already tested experimentally.
Acknowledgment
The authors thank to Institute of Industries of the Universidad del Mar for its financial support through the project CUP 2II1803. I.A.V. also thanks to CONACYT (México) for the scholarship granted (CVU 1017727).
References
- Chapin DM, Fuller CS, Pearson GL (1954) A new silicon p-n junction photocell for converting solar radiation into electrical power. J Appl Phys 25(5): 676.
- Zulehner W, Neuer B (1993) Silicon; Ullmann’s Encyclopedia of Industrial Chemistry. vol. A23, VHC Publishers Weinheim pp. 721-748.
- Kappaun S, Slugove C, List EJ (2008) Phosphorescent organic light-emitting devices: Working principle and iridium-based emitter materials. International Journal of Molecular Sciences 9: 1527-1547.
- Tang CW, Van Slyke SA (1987) Organic electroluminescent diodes. Applied Physics Letters 51(12): 913-915.
- Gharagheizi F, Llani-Kashkouli P, Acree WE, Mohammadi AH, Ramjugernath D (2013) A group contribution model for determining the sublimation enthalpy of organic compounds at the standard reference temperature of 298 K. Fluid Phase Equilibria 354: 265-285.
- Zhang Q, Chen J, Cheng Y, Wang L, Ma D, et al. (2004) Novel aromatic extended carbazoles as a chemical platform of bipolar hosts for improved lifetime in phosphorescent organic light-emitting diodes. Journal of Materials Chemistry 14: 895-900.
- Bao Nguyen QP, Baek SJ, Kim MJ, Shin NY, Kim GW, et al. (2014) Novel hole transporting materials based on 4-(9H-Carbazol-9-yl) triphenylamine derivatives for OLEDs. Molecules Journal 19: 14247-14256.
- Strohriegl P, Wagner D, Schrögel P, Sebastian T, Hoffmann ST, et al. (2013) Novel host for blue phosphorescent OLEDs. Organic Light Emitting Materials and Device XVII 8829: 1-5.
- Costa JCS, Santos LMNBF (2013) Hole transport materials based thin films: Topographic structures and phase transition thermodynamics of triphenylamine derivatives. J Phys Chem C 117(21): 10919-10928.
- El-ghandour A, Hameed MFO, Awed AS, Obayya A (2018) Optical and electrical properties of nanostructured N,N′-diphenyl-N,N′-di-p-tolylbenzene-1,4-diamine organic thin films. Applied Physics A 124(8): 124-542.
- Mendoza-Ruiz EA, Mentado-Morales J, Flores-Segura H (2019) Standard molar enthalpies of formation and phase changes of Tetra-N- phenylbenzidine and 4,40-Bis (N-carbazolyl)-1,10-biphenyl. J Therm Anal Calorim 135: 2337-2345.
- Mentado-Morales J, Ximello-Hernández A, Salinas-Luna J, Freitas VLS, Ribeiro da Silva MDMC (2008) A promising thermodynamic study of hole transport materials to develop solar cells.
© 2022 Javier Salinas-Luna and Juan Mentado-Morales. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






