- Submissions

Full Text
Polymer Science: Peer Review Journal
Nanofibers As Implantable Controlled Release Systems
Serim TM1, Eren-Boncu T2, Amasya G1, Sengel-Turk CT1 and Ozdemir AN1*
1Department of Pharmaceutical Technology, Faculty of Pharmacy, Ankara University, Turkey
2Department of Pharmaceutical Technology, Faculty of Pharmacy, Erciyes University, Turkey
*Corresponding author: Nurten Ozdemir, Ankara University, Faculty Pharmacy, Department of Pharmaceutical Technology, Ankara, Turkey
Submission: December 15, 2021;Published: January 20, 2022

ISSN: 2770-6613 Volume2 Issue5
Introduction
Controlled release systems that release the active substance at the desired rate for the desired time have been developed to reduce the incidence of side effects seen in treatment with classical dosage forms and to increase the compliance of the patients with the treatment by reducing the frequency of drug use. Controlled release systems are systems in which the release time and rate are adjusted according to the desired purpose, and the active substance can be targeted to a specific area of the body. Controlled release systems have many advantages over conventional systems such as improved efficacy, reduced toxicity, and better patient compliance [1]. The most ideal route of administration for controlled release systems is to implant dosage forms in the desired area of action. In the treatment with implantable controlled release systems; a) The treatment efficiency increases, and the side effects observed in the systemic drug delivery are eliminated as the delivery of the active substance is provided in the desired area, at the desired dose and for the desired time. b) Plasma concentration can be kept in the therapeutic range without fluctuation for a long time, since they release the active substance at a controlled rate. c) Problems seen in the absorption and distribution of the active substance in conventional dosage forms are eliminated. d) The risk of systemic side effects decreases as there will be a high concentration of active substance in the area of effect but very little active substance in other organs, since the implant is directly placed to the area where the therapeutic effect is desired. e) The possibility of forgotten doses decreases, patient compliance increases, auxiliary health personnel are not needed for drug intake, and treatment efficiency increases since there is no repetitive drug intake. Implantable delivery systems can be prepared in forms such as films, nanoparticles and microspheres, and the latest technology developed as implantable delivery systems is nanofiber technology.
Mini Review
Nanofibers are nontoxic and biocompatible drug delivery systems, that vary in size from nanometer to micrometer, have large surface area per unit mass compared to other pharmaceutical forms (for example, ~100 nm diameter nanofiber has a specific surface of 1000m2/g) and high surface-to-volume ratio, high porosity, superior flexibility and mechanical properties [2-5]. Compared to other dosage forms, they are systems that provide effective drug release due to their large surface area [6] and have higher encapsulation efficiency compared to spherical nanoparticles [6,7]. Nanofibers can be made into pharmaceutical dosage forms capable of rapid, delayed and controlled release. Nanofibers, which increase clinical efficacy by improving biopharmaceutical properties, also carry the advantages of classical solid dosage forms such as easy processability, stability and ease of transportation and storage [8-12]. Nanofibers, which provide cell growth and proliferation, are widely used in tissue engineering as medical prosthesis and tissue support, as well as in wound and burn treatment [13]. Nanofibers can be used in soft tissue prostheses such as vascular and breast, as well as adsorbed in the form of thin, porous structures on hard tissue prostheses implanted in the human body, and it is considered to be used as post-operative implants [6,14]. One of the biggest problems in the design of biomaterials to be applied to tissues is that these structures must be similar to the extracellular matrix [6]. Almost all human tissues and organs, including bone, teeth, cartilage, collagen and skin, are fibrous. In addition, cells perceive small and unsimilar materials as foreign matter and phagocytize them [3]. Due to being fibrous and nano-sized, electrospun nanofibrous structures can imitate the extracellular matrix, adapt to the tissue, are protected from phagocytosis, and trigger cell growth and proliferation with this size and shape [15]. In studies, fibers with diameters ranging from 3 nm to 1μm were produced with more than 50 polymers, and it has been shown that this method is suitable for both hydrophilic and hydrophobic active substances [6]. It is known that by implanting electrospun implants into the tissue, they provide localized, long-term, controlled release of active substance and are superior to the preparations in the market [16]. The study, which showed that nanofiber formulations produced for the delivery of tetracycline HCl with Polylactic Acid (PLA), Polyethylene Vinyl Acetate (PEVA) and Poly (Lactic Acid-Co-Glycolic Acid) (PLGA) 50:50 mixtures were superior to the commercial preparation, is one of the earliest promising studies to show that these systems can be used as drug delivery systems [17]. In studies, where it was observed that the formulations of sustained-release paclitaxelloaded PLGA electrospun nanofiber implants had an API release for 60 days in vitro and the formulations prepared for application to the tumor tissue in the brain in post-operative glioma chemotherapy had a controlled release for 24 days in vivo, it was proven that the nanofiber implant was superior to Taxol, which is the intravenous commercial product [16,18]. Nanofibrous structures have been formed with many polymers such as Poly-L-Lactic acid (PLLA), Polycaprolactone (PCL), PLGA and chitosan [13, 19-23].
The compatibility of the active ingredient/polymer/solvent is very important for the preparation of controlled release nanofibers. In the study, in which electrospun PLLA fibers were produced with lipophilic paclitaxel, hydrophilic doxorubicin hydrochloride and lipophilic doxorubicin base, it has been found to have good encapsulation and release with zero-degree kinetics since paclitaxel is compatible with PLLA and dissolves in chloroform/acetone solvent [24]. A polymer suitable for the active substance must be used in order to encapsulate most of the active substance into the fiber and to provide a continuous and stable release profile, and the solvent used must be compatible with both the polymer and the active substance [24]. When nanofiber formulations with different antibiotics were implanted, antibiotics were observed above the minimal inhibition concentration around the implantation site for a long time, while the concentration in the bloodstream was quite low and the risk of side effects seen when antibiotics were given by other administration routes was reduced [25,26]. There are also studies on the use of antibiotic-loaded nanofibers for local effect by adsorbing them to vascular grafts or prostheses on the market [25,27]. In these studies, while most of the active substance is encapsulated into the material, some adsorbing to the surface causes the burst release, and it has been observed that it has a positive effect in the treatment of bacterial infections by allowing microorganisms to be exposed to high doses of antibiotics at the beginning [27,28]. As a result of in vitro and in vivo experiments, although there is very little active substance left in the adsorbed material and on its surface, the presence of a high concentration of active substance in the tissues around the material has proven that local effect is achieved by preventing the systemic side effects of the active substance [27]. It has also been observed that metronidazole/ PCL nanofibers reduced inflammation compared to PCL nanofibers when applied as a subcutaneous implant, inhibited anaerobic bacterial growth and maintained these effects for 2 weeks [29].
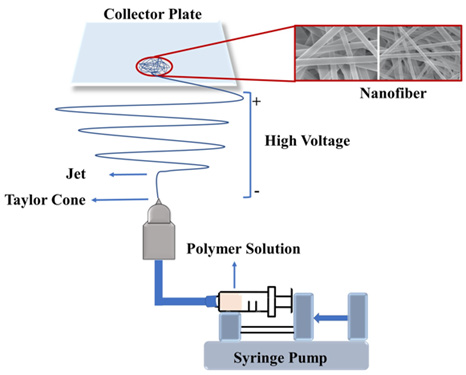
There are many methods for the production of polymeric nanofibers. These are: The drawing technique, template synthesis, phase separation, self-assembly and electrospinning. Electrospinning was patented in 1934 and applied in many fields, especially tissue engineering [13]. Among the nanofiber preparation methods, electrospinning method was determined as the most suitable method because products with a wide range of fiber diameters can be obtained uniformly by changing the formulation and system parameters, it is a one-step method that does not require expensive purification processes and it provides reproducible data, it is suitable for scale-up, and it allows production with many polymers and solvents [6,30,31]. Electrospinning is a method of increasing interest in tissue engineering and controlled drug release globally since it can form structures from micro to nano size with high porosity, resembling the extracellular matrix. This method basically requires 3 components:
a. High voltage source (around 30kV)
b. Needle
c. Collector metal plate
While performing the electrospinning process; The polymer solution is filled into the injector and connected to the electrospinning needle after the injector is placed in the pump. The distance between the needle and the collector plate is adjusted and high voltage is applied between the needle tip and the collector plate with the help of the power supply. When the system reaches the critical voltage value, the polymer solution is sprayed from the injector. The droplet at the needle tip is deformed due to the electrostatic attraction of the counter electrode just before the surface tension is overcome. The cone shape formed at the needle tip of the drop is called the “Taylor Cone” [32,33]. The polymer solution that reaches this value is electrically charged positively and moves towards the negatively charged collector. Meanwhile, the solvent in the polymer solution evaporates, forming structures called electrospun fibers with very fine fibers on the metal collector plate. The critical parameters in the electrospinning process are:
a. Selection of the appropriate solvent system for the
polymer,
b. Having an appropriate solution viscosity and surface
tension,
c. Determining the flow rate that will enable the Taylor cone
to form,
d. Application of the optimum voltage,
e. Setting of the appropriate distance for the solvent
evaporation [2,31,34].
In the production of electrospun polymeric fiber, the physical and mechanical properties of the nanofiber and its chemical properties, including the degradation rate, can be changed by optimizing the parameters such as voltage, polymer-solvent systems, distance between the injector and the collector plate, pump speed, injector inner diameter, etc. Nanofibers with desired properties can be produced with many materials, including polymers, biopolymers, DNA, proteins, and even macromolecules such as phospholipids. The illustration of electrospinning method is given in Figure 1.
Figure 1: Schematic illustration of the electrospinning method.
Conclusion
In recent years, the use of polymeric nanofibers has gained great importance in biomedical and biotechnological applications such as tissue engineering, controlled release systems, wound dressings, medical implants, composites for dental applications and biosensors. Among the different nanofiber preparation methods, the electrospinning method is the most preferred production method because it allows the production of nanofibers with different materials, from natural or synthetic polymers to ceramics, and allows cheap, simple and fast production. Polymeric nanofibers are promising drug delivery systems, especially in terms of their applications as controlled release systems to achieve localized drug delivery.
References
- Uhrich KE, Cannizzaro SM, Langer RS, Shakesheff KM (1999) Polymeric systems for controlled drug release. Chem Rev 99(11): 3181-3198.
- Frenot A, Chronakis IS (2003) Polymer nanofibers assembled by electrospinning. Cur Opin Coll Int Sci 8(1): 64-75.
- Huang ZM, He CL, Yang A, Zhang Y, Han XJ, et al. (2006) Encapsulating drugs in biodegradable ultrafine fibers through co-axial electrospinning. J Biomed Mater Res A 77(1): 169-179.
- Wagh A, Singh J, Qian S, Law B (2013) A short circulating peptide nanofiber as a carrier for tumoral delivery. Nanomedicine 9(4): 449-457.
- Zhang Q, Li Y, Lin ZYW, Wong KKY, Lin M, et al. (2017) Electrospun polymeric micro/nanofibrous scaffolds for long-term drug release and their biomedical applications. Drug Discov Today 22(9): 1351-1366.
- Huang ZM, Zhang YZ, Kotaki M, Ramakrishna S (2003) A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos Sci Technol 63(15): 2223-2253.
- Yu DG, Zhu LM, White K, Branford WC (2009) Electrospun nanofiber-based drug delivery systems. Health 1(2): 67-75.
- Carracciolo PC, Cortez TPR, Ballarin FM, Abraham GA (2013) Development of electrospun nanofibers for biomedical applications: State of The Art in Latin America J Biomater Tissue Eng 3: 39-60.
- Cui W, Zhou Y, Chang J (2010) Electrospun nanofibrous materials for tissue engineering and drug delivery. Sci Technol Adv Mate 11(1): 014108.
- Kenawy ER, Abdel HFI, El Newehy MH, Wnek GE (2009) Processing of polymer nanofibers through electrospinning as drug delivery systems. Mater Chem Phys 113(1): 296-302.
- Nalwa HS (2000) Handbook of Nanostructured Materials and Nanotechnology. Academic Press, San Diego, USA.
- Ramalingam M, Ramakrishna S, Rutledge G (2013) A special section on advances in electrospinning of nanofibers and their biomedical applications. J Nanosci Nanotechnol 13: 4645-4646.
- Li WJ, Laurencin CT, Caterson EJ, Tuan RS, Ko FK (2001) Electrospun nanofibrous structure: A novel scaffold for tissue engineering. J Biomed Mater Res 60(4): 613-621.
- Eren-BT, Uskudar GA, Catma MF, Savaser A, Gokce A, et al. (2020) In vitro and in vivo evaluation of linezolid loaded electrospun PLGA and PLGA/PCL fiber mats for prophylaxis and treatment of MRSA induced prosthetic infections. Int J Pharm 573: 118758.
- Dalton PD, Lleixa CJ, Mourran A, Klee D, Moller M (2006) Melt electrospinning of poly-(ethylene glycol-block-epsilon-caprolactone). Biotechnol J 1(9): 998-1006.
- Ranganath SH, Wang CH (2008) Biodegradable microfiber implants delivering paclitaxel for post-surgical chemotherapy against malignant glioma. Biomaterials 29(20): 2996-3003.
- Kenawy ER, Bowlin GL, Mansfield K, Layman J, Simpson DG, et al. (2002) Release of tetracycline hydrochloride from electrospun poly(ethylene-co-vinylacetate), poly(lactic acid), and a blend. J Control Release 81(1-2): 57-64.
- Xie J, Wang CH (2006) Electrospun micro- and nanofibers for sustained delivery of paclitaxel to treat C6 glioma in vitro. Pharm Res 23(8): 1817-1826.
- Bhattarai N, Edmondson D, Veiseh O, Matsen FA, Zhang M (2005) Electrospun chitosan-based nanofibers and their cellular compatibility. Biomaterials 26(31): 6176-6184.
- Kim K, Fisher JP (2007) Nanoparticle technology in bone tissue engineering. J Drug Target 15(4): 241-252.
- Santos MI, Tuzlakoglu K, Fuchs S, Gomes ME, Peters K, et al. (2008) Endothelial cell colonization and angiogenic potential of combined nano- and micro-fibrous scaffolds for bone tissue engineering. Biomaterials 29(32): 4306-4313.
- Tuzlakoglu K, Bolgen N, Salgado AJ, Gomes ME, Piskin E, et al. (2005) Nano- and micro-fiber combined scaffolds: A new architecture for bone tissue engineering. J Mater Sci Mater Med 16(12): 1099-1104.
- Woo KM, Jun JH, Chen VJ, Seo J, Baek JH, et al. (2007) Nano-fibrous scaffolding promotes osteoblast differentiation and biomineralization. Biomaterials 28(2): 335-343.
- Zeng J, Yang L, Liang Q, Zhang X, Guan H, et al. (2005) Influence of the drug compatibility with polymer solution on the release kinetics of electrospun fiber formulation. J Control Release 105(1-2): 43-51.
- Hsu YH, Chen DW, Tai CD, Chou YC, Liu SJ, et al. (2014) Biodegradable drug-eluting nanofiber-enveloped implants for sustained release of high bactericidal concentrations of vancomycin and ceftazidime: in vitro and in vivo Int J Nanomedicine 9: 4347-4355.
- Karuppuswamy P, Reddy VJ, Navaneethan B, Luwang LA, Ramakrishna S (2015) Polycaprolactone nanofibers for the controlled release of tetracycline hydrochloride. Mater Lett 141: 180-186.
- Liu KS, Lee CH, Wang YC, Liu SJ (2015) Sustained release of vancomycin from novel biodegradable nanofiber-loaded vascular prosthetic grafts: in vitro and in vivo Int J Nanomedicine 10: 885-891.
- Liu KS, Liu SJ, Chen HY, Huang YK, Peng YJ, et al. (2012) Steady antibiotic release from biodegradable beads in the pleural cavity: an in vitro and in vivo Chest 141(5): 1197-1202.
- Xue J, He M, Liang Y, Crawford A, Coates P, et al. (2014) Fabrication and evaluation of electrospun PCL–gelatin micro-/nanofiber membranes for anti-infective GTR implants. J Mater Chem B 2(39): 6867-6877.
- Beachley V, Wen X (2010) Polymer nanofibrous structures: Fabrication, biofunctionalization, and cell interactions. Prog Polym Sci 35(7): 868-892.
- Pelipenko J, Kocbek P, Kristl J (2015) Critical attributes of nanofibers: preparation, drug loading, and tissue regeneration. Int J Pharm 484(1-2): 57-74.
- Guo-ES, Hong T, Chun-LZ, Yan-Li D, Yi L (2010) Preparation of ultrafine water-soluble polymers nanofiber mats via electrospinning. Chem Res Chinese U 26(2): 318-322.
- Lin T, Wang H, Wang H, Wang X (2004) The charge effect of cationic surfactants on the elimination of fibre beads in the electrospinning of polystyrene. Nanotechnology 15(9): 1375-1381.
- Ignatious F, Baldoni J (2003) Electrospun pharmaceutical compositions. USA.
© 2022 Ozdemir AN. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






