- Submissions

Full Text
Polymer Science: Peer Review Journal
Development of Polymers Coated with Immobilized Carbonic Anhydrase for CO2 Capture and its Application
Xiaobo Li1,2, Ke Li2,3, Haoran Yang2,3,*, Peiqing Li2,3, Bohan Shen2,3, Zhigang Wang2,3, Kaihua Xiao2,3, Qiuyan Chen2,3, Chang Su2,3, Hao Huang2,3 and Diming Lou1
1Automotive college, Tongji University, China
2R&D center, Shanghai Marine Diesel Engine Research Institute, China
3National Engineering Laboratory for Marine and Ocean Engineering Power System, Shanghai Marine Diesel Engine Research Institute, China
*Corresponding author: Haoran Yang, Shanghai Marine Diesel Engine Research Institute, No.1, Lane357, Gongqing Road, Shanghai, China
Submission: December 20, 2021;Published: January 17, 2022

ISSN: 2770-6613 Volume2 Issue5
Abstract
The rocketing CO2 emissions has caused intensified global warming effect to human beings. Although several approaches have been proposed to reduce atmospheric CO2 emissions, high temperature condition and high energy consumption make those approaches hard to carried out in industrial application. Carbonic Anhydrase (CA) catalyzed hydration reactions or aqueous solvents are a potential alternative in conventional amine-based CO2 capture, for its energy saving and excellent performance. However, free CA will significantly increase the CA amounts and system cost, as well as decreasing the system efficiency. To face the mentioned issues, some new methods of immobilization of CA are introduced, which can help to provide new ideas for alleviating greenhouse effect and global warming.
Keywords: Polymer; Carbonic anhydrase immobilization; CO2 capture; Greenhouse effect control
Introduction
Human beings have been lived on fossil fuels, like oil, coal and nature gas, since the Industrial Revolution, leading to sharping increasing of atmospheric greenhouse gas [1]. The increasing greenhouse gas in atmosphere has aroused severe global warming and threatened creature livings on Earth. To alleviate greenhouse effect and global warming, many governments and organizations have taken action to reduce artificial greenhouse gas emission, especially CO2 [2]. Research show that amine-based chemical absorption technology [3] is the most prevailing approach in CO2 control. However, it is also pointed out that aminebased chemical absorption technology not only needs intensified energy to regenerate but leads to degrade and toxicity issue. As a result, amine-based chemical absorption technology might not seem like the best solution for CO2 control.
Recently, CA catalyzed CO2 control technology have entered scholars’ vision [4]. Due to CA’s activity, it can catalyze CO2 hydration reactions, greatly improving CO2 absorption rate than those non- catalyzed. Besides, its end products are very stable and are environmentally friendly. Therefore, CO2 capture using CA is the most promising method at present. But CA also has its one disadvantages. Free CA is a kind of vulnerable enzyme and easily turns to deactivated under extreme working condition, like high temperature and amine environment [5]. What’s more, free CA is also hard to recover and recycle. Therefore, immobilized CA on certain material can greatly improve CA performance and lower cost of whole system. This review describes several methods, immobilizing CA on certain polymer materials, that improving CA performance. Those methods in improving CA performance will become a prevailing way in application.
Discussion
Polyurethane (PU)
Bovine Carbonic Anhydrase (BCA) was immobilized within PU foam, and the kinetic parameters of the immobilized enzyme Km decreased from 12.2mmol/L to 9.6mmol/L, compared with the free enzyme [6]. However, the thermal stability of the immobilized enzyme was significantly enhanced (Figure 1). After 7 cycles of continuous operation in the reactor or 45 days of storage at room temperature, the enzyme activity remained 100%, while the free enzyme was completely inactivated even after 45 days of storage at 4 °C. There was also other paper pointed out that immobilized SspCA on PU foam had an excellent thermal stability It could survive for 48h at 100 °C and work for a long time at 70 °C and could be used in the reactor of high-temperature CO2 capture in thermal power plants [7].
Figure 1: Performance of immobilized CA on PU foam:(a) SEM image of the PU foam, (b) Cycle of usage for CA immobilized within PU foam, (c) Stability of the free (at 4 °C) and immobilized (at room temperature) CA in Tris buffer.

Chitosan
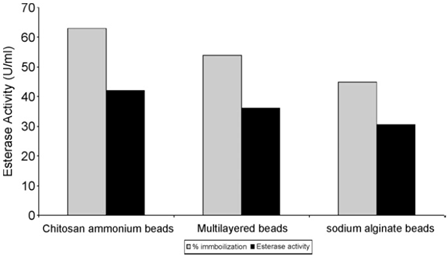
CA enzyme from Bacillus Pumilus could be immobilized on the surface of chitosan at room temperature, and the stability of the immobilized enzyme to heat and inhibitor was improved [8]. After holding at 50 °C for 90 days under the same conditions, the immobilized enzyme could maintain 60% activity of the initial enzyme, while the free enzyme was only 30%. Meanwhile, the precipitation rate of CaCO3 was twice as that of free enzyme within 5min (Figure 2).
Figure 2: Percent immobilization and esterase activity of Bacillus pumilus on matrices.
Nanoparticles
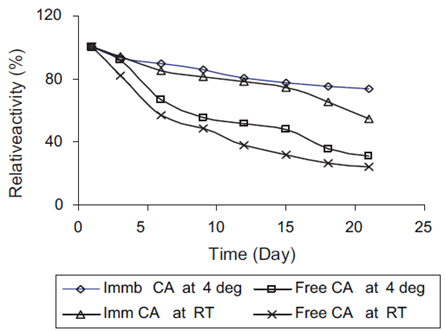
Bovine Carbonic Anhydrase (BCA) could be covalently immobilized onto chemically treated Fe3O4/SiO2 nanoparticles by using glutaraldehyde as a spacer [9]. The CO2 capture capacity of the immobilized enzyme was 26 times that of the free enzyme after 30 cycles in the reactor. The immobilized enzyme could retain 82% activity of the original enzyme after 30-day storage at room temperature (Figure 3). The magnetic nano biocatalyst was shown to be an excellent reusable catalyst for CO2 capture. Besides chemically treated nanoparticles, alginate nanoparticle was another option for CA immobilization [10]. The immobilized CA showed a better operational stability, retaining nearly 67% of its initial activity even after six cycles. Besides, the microspheres with the smallest particle size had the highest enzyme activity. What’s more, compared with free enzyme, the thermal stability of immobilized enzyme was significantly enhanced, and its optimal temperature was increased by nearly 10 °C (Figure 4).
Figure 3: Performance of immobilized CA on nanoparticles (a) HRTEM images of Fe3O4 and Fe3O4/SiO2/OAPS, (b) Optimization of control experiments, (c) Reusability of Fe-CA, (d) the effect of storage stability.
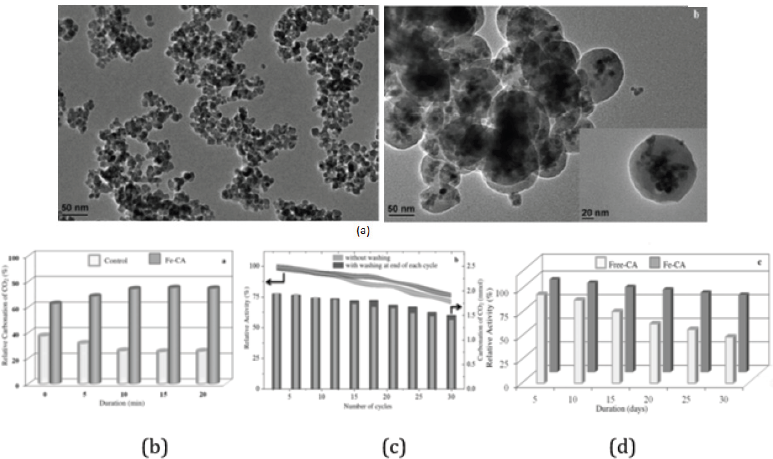
Figure 4: Stability profile of CA at various conditions.
Conclusion
In conclusion, although CA is potential catalyst in CO2 capture, the stability and reusability of CA is still too poor for application. Much research has confirmed that immobilized CA on certain material can greatly improve CA performance and lower cost of whole system, especially some polymer material. Polyurethane, chitosan and even nanoparticles are possible immobilization material for CA and those immobilization material can greatly improve CA performance in CO2 capture, which can greatly promote the industrial application of CA-based CO2 capture.
References
- Abokyi E, Appiah KP, Abokyi F, Oteng AEF (2019) Industrial growth and emissions of CO2 in Ghana: The role of financial development and fossil fuel consumption. Energy Rep 5: 1339-1353.
- Awaworyi CS, Inekwe J, Ivanovski K, Smyth R (2021) Transport infrastructure and CO2 emissions in the OECD over the long run. Transport Res D: Tr E 95: 102857.
- Caporaso JG, Lauber CL, Walters WA, Berg LD, Huntley J, et al. (2012) Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. The ISME J 6(8): 1621-1624.
- Chang S, He Y, Li Y, Cui X (2021) Study on the immobilization of carbonic anhydrases on geopolymer microspheres for CO2 J Cleaner Prod 316: 128163.
- Karlsson HK, Makhool H, Karlsson M, Svensson H (2021) Chemical absorption of carbon dioxide in non-aqueous systems using the amine 2-amino-2-methyl-1-propanol in dimethyl sulfoxide and N-methyl-2-pyrrolidone. Sep Purif Technol 256: 117789.
- Ozdemir E (2009) Biomimetic CO2 sequestration: 1. immobilization of carbonic anhydrase within polyurethane foam. Energy Fuels 23(11): 5725-5730.
- Prabhu C, Wanjari S, Gawande S, Das S, Labhsetwar N, et al. (2009) Immobilization of carbonic anhydrase enriched microorganism on biopolymer-based materials. J Mol Catal B: Enzym 60(1-2): 13-21.
- Rasouli H, Iliuta I, Bougie F, Garnier A, Iliuta MC (2021) Enhanced CO2 capture in packed-bed column bioreactors with immobilized carbonic anhydrase. Chem Eng J 432: 134029.
- Yadav RR, Mudliar SN, Shekh AY, Fulke AB, Devi SS, et al. (2012) Immobilization of carbonic anhydrase in alginate and its influence on transformation of CO2 to calcite. Process Biochem 47(4): 585-590.
- Vinoba M, Bhagiyalakshmi M, Jeong SK, Nam SC, Yoon Y (2012) Carbonic anhydrase immobilized on encapsulated magnetic nanoparticles for CO2 sequestration. Chem - Eur J 18(38): 12028-12034.
© 2022 Haoran Yang. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






