- Submissions

Full Text
Polymer Science: Peer Review Journal
Effect of Cellulose-g-Poly(L-Lactide) on the Properties of Woody Thin Boards Made of Lignin/Cellulose Biphasic System: Water Repellency and Long-Term Stability
Toshinori Shimanouchi1, Masafumi Yoshida1, Wei Yang2 and Yukitaka Kimura1*
1Graduate School of Environmental and Life Science, Okayama University, Japan
2College of Materials and Environmental Engineering, Hangzhou Dianzi University, China
*Corresponding author: Yukitaka Kimura, Graduate School of Environmental and Life Science, Okayama University, 3-1-1 Tsushimanaka, kita-ku, Okayama, Okayama 700-8530, Japan
Submission: September 22, 2021;Published: September 30, 2021

ISSN: 2770-6613 Volume2 Issue3
Abstract
Cellulose was grafted with Poly(L-lactide) (PLLA) to synthesize cellulose-g-PLLA in this study, which improved the water repellency of woody thin boards made of cellulose and lignin, prepared by a compression molding. It was clarified, from the diagram study of ternary cellulose/lignin/water phase system, that the content of cellulose-g-PLLA was crucial to form WTBs. The best performance of water repellency for WTBs was 126deg of the contact angle (30 % content of cellulose-g-PLLA), as compared with 50deg for bare WTB. This was likely to result from the hydrophobicity of PLLA modified at the surface of cellulose particles. Moreover, the stability of WTBs for six years was improved by celluloseg- PLLA. The improved stability can be explained by an entanglements between PLLA polymer chains or between PLLA and lignin.
Keywords: Cellulose-g-PLLA; Water repellency; Long-term stability; Woody thin board
Introduction
Woody biomass such as cellulose, hemicellulose, and lignin are expected to be used in the chemical conversion and mechanical manufacturing to the value-added materials. One of the applications is Wood Plastic Composites (WPCs) that are produced by thoroughly mixing wood particles and thermoplastic resin are a promising material to utilize the woody biomass [1-12]. It has been reported that the mixture of cellulose and lignin could yield the Woody Thin Boards (WTBs) by a compression molding [13]. Accordingly, WPCs and WTBs favor the biodegradative property, which is preferable to reuse. It is, therefore, expected that both are utilized in the field of building. Besides, the biomass energy can be stored as the WPCs and WTBs if their shelf time would surpass the growth time of woods, which might contribute to the carbon neutral process for green chemistry. For this purpose, WPCs and WTBs should be stable during the growth of wood. Therefore, the surface modification of WPCs by additives is available to improve the mechanical and chemical stability. The conventional WPCs composes of woody materials (80~90 % in weight) and additives (10~20 % in weight) for an improvement of functionality with respect to the mechanical property [2-12], the water repellency [2,3], and the surface geometry [3-5]. For examples, the additives used for the improvement of mechanical strength of WPCs are fire retardant [3], lubricant [8,9], polymers [4-7,10], and coupling agents [8,9]. The chemical treatment such as acetylation [11] and silane treatment [12] has also been developed to improve the mechanical strength of WPCs. Thus, the prolonged effect on the shelf time of WPCs and WTBs has been developed. Specifically, the water-resistant property is indispensable for the functional materials from woody biomass. Cellulose is easily to be swollen and often lead to depolymerization [14], which cause their deterioration. Then, the water repellency of WPCs and WTBs should be improved. The water repellency has mainly been improved by the microscopic roughness of the surface [15] and the chemical approach [16]. There has been a small number of reports along the chemical approach. If the surface property of WPC and WTBs would be clarified, such finding would give a better understanding on the surface modification.
In recent reports, the improved hydrophobicity would be helpful for the water repellency [13,16]. The induction of hydrophobic polymer is a promising approach [17]. Poly(lactide) (namely PLLA in this article), yielded from lactide or lactic acid, is the popular compound. It is noted that lactide or lactic acid is recognized as a value-added materials in chemical conversion process from biomass. A graft polymerization of PLLA to cellulose (cellulose-g-PLLA) has been reported [18]. Cellulose-g-PLLA is a new biodegradable copolymer comprised of hydrophobic PLLA segments and hydrophilic cellulose segment, which is a promising compound in terms of green chemistry and sustainable development [18]. Polymer chain shows the entanglement to give a certain impact on the adhesive property of material interfaces [19]. Accordingly, cellulose-g-PLLA might affect not only water repellency but also the mechanical property of WTBs such as the formation property of WTBs and their long-term stability. Therefore, the investigations mentioned above would be helpful to deeply understand the formation mechanism of WTBs with a compression molding. Besides, a use of PLLA would be a promising application of glycerol as a by-product of the biofuel because a production process of lactic acid from glycerol has been well established [20]. In this study, we aimed the chemical modification of WTBs using cellulose-g-PLLA according to the previous method [18]. The diagram of cellulose/lignin/water+cellulose-g-PLLA was made from the WTB formation test. We compared the efficacy of chemical modification of WTBs with dipping method, in terms of water repellency. Furthermore, the long-term stability of WTBs was examined using the contact angle.
Materials and Methods
Materials
Purified lignin, cellulose (crystalline cellulose I) and poly(Llactide) (PLLA, Mw. 10,000) were purchased from Wako Pure Chemical Ltd. (Hiroshima, Japan). The average diameter of lignin and cellulose was 3 and 20mm, respectively. L-Lactide was purchased from Funakoshi Co. Ltd. (Tokyo, Japan). Other reagents were of analytical grade.
Preparation of WTBs by the compression molding
Lignin and cellulose were mixed with a certain composition ratio for 20min. Water (0~40mg) was thereafter added to mixture (30mg) and milled for 2min. The above mixture was injected into the metal mold and the compression molding was performed at 180 °C and 25MPa for 10min. Afterwards, the mold was considerably cooled down to recover woody thin boards with 10mmx10mm in size; 0.3mm in depth.
Synthesis of cellulose-graft-PLLA for chemical modification of WTBs
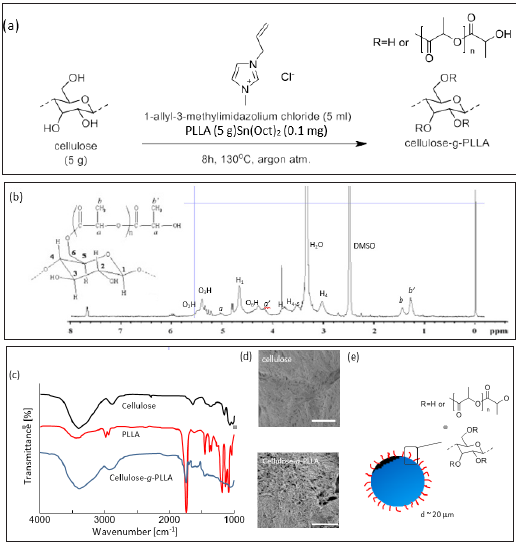
Cellulose-g-PLLA was synthesized according to the previous report [18]. Lactide (5g) was polymerized by using 0.1mg of Sn(Oct)2 as catalyst, by a ring-opening polymerization reaction. Polymerization reaction was conducted at 130 °C for 8 hours in argon atmosphere. Cellulose (5g) was mixed with PLLA (5g) in the presence of 1-allyl-3-methylimidazolium chloride (5ml). This mixture was incubated under argon atmosphere at 130 °C for 8h. Afterwards, both the solution obtained, and catalyst were removed with ethanol. Thereafter, cellulose unreacted was separated by chloroform (mixing for 72h was required). The obtained sample was filtrated with chloroform to separate the residue and dried for 48h in vacuum. Finally, 1H-NMR and FTIR measurements were performed to determine the chemical structure of the sample. For 1H-NMR measurement, the samples were filtrated and rinsed by ethanol three times to remove the reaction solvent and catalyst. The residues were mixed with dichloromethane and thereafter stirred for 72 hours at room temperature to remove the unreacted cellulose. Further rinsing by dichloromethane several times were performed to obtain the measurement sample. DMSO-d6 was used for the solvent of 1H-NMR. For the FTIR measurement, the instrument used was an FT/IR-4300ST equipped with an Attenuated Total Reflactometry (ATR) unit (ZnSe crystal) (Nihon Bunko Co., Ltd., Tokyo, Japan), according to the literature [13]. Cellulose, PLLA, and cellulose-g-PLLA was measured with a resolution of 4cm-1 and an accuracy of the frequency reading of ±0.4cm-1. ZnSe crystal can give the analytical space with 100nm in depth from the surface.
Dipping method
WTBs were dipped in a commercial PLLA solved in chloroform solution. Subsequent drying at 100 °C for one day was performed to obtain the sample.
Measurement of contact angle
The contact angle of water on samples was measured by using an auto-contact angle/surface tensiometer (DSA 10, Krüss, Switzerland). The image of the water drop was recorded and analyzed with data acquisition software after 1min. Then, the contact angle was measured by drawing a line at the edge of the water drop. For the long-term stability experiment, ten WTBs were settled on the sheet at room temperature (20~27 °C). aliquot of water droplet was poured onto each WTB half-by-half year to measure the contact angle in air. Afterwards, water droplet was eliminated by a piece of paper. WTB cracked was ruled out to monitor the survival ratio of WTBs.
.Scanning electron microscopy (SEM)
The morphology of samples was observed by using a scanning electron microscope (SEM) (S-4700, Hitachi Ltd., Tokyo, Japan). Samples were mounted on metal stubs by double-faced tap and images were taken. Prior to imaging samples were coated with platinum-palladium in a sputter coater (E1030 Sputter, Hitachi Ltd.).
Other measurements
The combustion analysis was conducted on a differential thermal analyzer Thermo plus TG8120 (Rigaku, Tokyo, Japan). The experiment was carried out at the temperature range from room temperature to 800 °C with the heating rate of 20 °C/min under air atmosphere (100mL/min). The mean diameter of cellulose particles was measured using a dynamic light scattering mode of FPAR (Ohtsuka Electronics Co. Ltd., Japan).
Results and Discussion
Synthesis and characterization of cellulose-g-PLLA
Cellulose-g-PLLA was prepared according to the previous report [18], i.e. the hydrogen of hydroxyl group in glucose was replaced by PLLA in the presence of 1-allyl-3-methylimidazolium chloride to obtain the target compound. The chemical reaction is shown in Figure 1a. For a confirmation of cellulose-g-PLLA synthesis, the 1H-NMR chart is shown in Figure 1b. Peaks not only from glucose ring but also PLLA were observed. Overall, the NMR chart obtained here was consistent with the previous report [18]. Furthermore, FT-IR measurement was performed to confirm the induction of PLLA to cellulose. ATR-FTIR is one of the most powerful techniques to detect the chemical composition to 100nm deep from a surface. We compared the FTIR spectra for cellulose, PLLA, and cellulose-g- PLLA. The strong peak at 3400cm-1 is assigned to the OH stretching mode and new band appears at about 1750cm-1 is assigned to the carbonyl group [18]. These peaks were also observed only in PLLA grafted on cellulose, not in homo-PLLA (bare PLLA). The above two mentioned peaks exist in cellulose-g-PLLA. These results suggested the synthesis of the graft copolymer on the surface of cellulose particles, which was consistent with the report [18]. The average diameter of cellulose-g-PLLA was measured with DLS measurement. No definitely difference in diameter between cellulose modified and unmodified with PLLA was observed (20mm in average diameter). Thermal gravimetric analysis indicated the no decrease in weight of samples in the temperature range, i.e. a softening temperature 240 °C [21]. Thus, both DLS and thermal gravimetric measurements suggested no progression of either pyrolysis or depolymerization of cellulose during the modification reaction with PLLA under the reaction temperature of 130 °C.
SEM observation was performed to confirm the variation of the surface of the cellulose particles during the reaction shown in Figure 1a. Before the reaction, microfibrils of cellulose could be observed. After the reaction, the disturbed surface was observed without the significant change of cellulose particles due to the pyrolysis or depolymerization of cellulose. It was therefore considered that the cellulose surface was modified with PLLA without a significant reduction of particle size (Figures 1c-1e).
Figure 1: (a) Synthesis of cellulose-g-PLLA for chemical modification. (b) 1H-NMR chart. DMSO-d6 was used as the solvent. (c) FTIR spectra for cellulose, PLLA, and cellulose-g-PLLA. (d) SEM images of the surfaces of cellulose and cellulose-g-PLLA. White bars represent 500nm. (e) Schematic illustration of cellulose particle modified with PLLA.
WTB formation using cellulose-g-PLLA
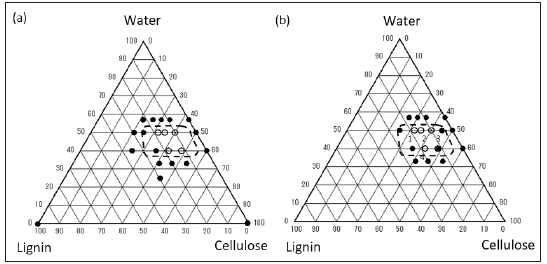
The preparation of WTBs was attempted by changing the composition of lignin, cellulose, and water under the constant ratio of cellulose-g-PLLA to cellulose (0 to 50wt%). First, 40 and 50% of cellulose-g-PLLA content made it impossible to form WTBs (data not shown). When the content of cellulose-g-PLLA was ranged between 0 and 30%, WTBs were obtained. Figure 2 shows the relationship between the preparation of WTBs and their composition. In the case of 0 % of cellulose-g-PLLA, the range for WTB formation were indicated as the region closed by a broken curve in Figure 2a. In addition, the use of cellulose-g-PLLA (20 and 30wt%) shrank the region with an increase in content of cellulose-g-PLLA, as shown in Figures 2a & 2b. These results suggested the excess mixing of cellulose-g-PLLA being disadvantageous for WTB formation. As a common point between (Figures 2a & 2b), samples in the powderlike aggregates including much moisture were obtained at the water content greater than 50%. The compression molding for 10min at 180 °C was obviously not enough to achieve the dewatering. In the case of 40~50% of water content, woody thin boards were obtainable at the similar composition ratios to the bare WTB [13]. When the water content was less than 40%, the obtained samples were in powdered-state, not in thin board-state. The same was true for the compression molding without water. It would be necessary that the additive effect of cellulose-g-PLLA on the WTB formation should be separately discussed from both the water content and ratio of lignin/cellulose. Thus, the induction of PLLA layer on cellulose particles gave the impact on the compression molding of WTBs.
Figure 2: Lignin-cellulose-water phase diagram. Content of cellulose-g-PLLA to cellulose was fixed at (a) 20wt% and (b) 30wt%. Closed key: unformable condition; open key: formable condition. The region enclosed by the broken curve is the formable region at 0wt%, as previously reported [13].
Effect of chemical modification on water repellency
Figure 3: (a) Contact angle of some WTBs. The weight percentage of cellulose-g-PLLA was 30%. Entries are denoted in Figure 2(b). (b) Effect of cellulose-g-PLLA on water repellency of WTB. Standard errors were calculated from the different four experiments. Water (30mg), cellulose (21mg), and lignin (9mg) was used to prepare WTBs. Previous study [13] demonstrated that lignin particles oriented at the surface of WTBs.
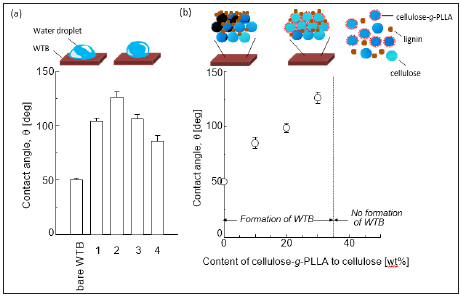
The orientation of PLLA to WTBs should affect the water repellency. Accordingly, the contact angle of WTBs tested in Figure 2b was estimated. The large contact angle of water droplets on the surface means that the surface indicates the strong water repellency [2,3,13,15]. Figure 3a demonstrated that the contact angle for entries 1-4 and bare WTB. Their contact angle depended on the composition. Apparently, the contact angle of entry 2 was estimated to be 126deg, which was the highest value tested here. The influence of the content of cellulose-g-PLLA on the contact angle of WTBs was then investigated. The ratio of cellulose-g-PLLA to cellulose was varied between 0 and 50wt% under the condition where the total cellulose content was constant: water 30mg, total cellulose 21mg, and lignin 9mg. As shown in Figure 3b, the contact angle monotonously increased by increasing the ratio of celluloseg- PLLA up to 30wt%. The contact angle above 30wt% was not measurable due to the unformable condition of WTBs as mentioned in the last section. It was therefore concluded that cellulose-g-PLLA is obviously oriented into the surface of WTB.
Effect of cellulose-g-PLLA on the stability of WTBs
Figure 4:Long-term stability of WTBs. (a) contact angle and (b) remaining ratio of WTBs. Initially, ten WTBs were prepared. Standard errors were calculated from the different four experiments in each WTB. “Chemical modification” is a WTB using cellulose-g-PLLA (entry 2). “Dipping” is a PLLA-dipped WTB.
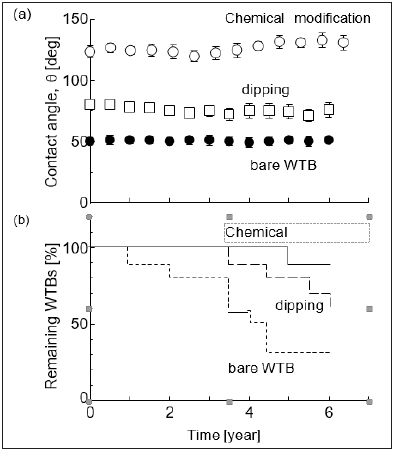
A comparison of surface modification of WTB with PLLA was examined by using a chemical modification by means of cellulose-g- PLLA and dipping method. Figure 4a shows the long-term stability concerning the water repellency. Initially, a contact angle was 80deg for PLLA-dipped WTB and 126deg for chemical modification method (entry 2), respectively. The chemical modification method indicated the highest value of contact angle of three methods. It was therefore likely that the WTB surface indicated the weak adhesive force to PLLA. This result suggested the efficacy of chemical modification of PLLA to WTBs. Furthermore, the long-term stability of entry 2 was examined in Figure 4a. The water repellency for chemicallymodified WTB (entry 2) and bare WTB kept almost constant for six years. Together with this, the survival WTBs was monitored in Figure 4b. First, The order of decremental number of WTBs was bare WTBs>WTBs dipped with PLLA>chemically-modified WTB. Second, 90% of chemically-modified WTB, 60% of WTBs dipped with PLLA, and 30% of bare WTBs could survive during six years. Thus, addition of cellulose-g-PLLA gave an impact of not only water repellency but also the stability of WTBs.
Discussion
A possible mechanism on the compression molding of WTB consists of three steps (Figure 5): i.e. the packing of polymer particles as the first step; softening polymers as the second step; and the adhesive as the third step. The first step is a packing of cellulose and lignin particles with different diameters. In the earlier study [22], a simple model was proposed for estimating the void fraction, which is an important property of the particle bed. This study said that a void fraction minimized in the 20~30% of the mixture ratio of smaller particle to a larger one, whose diameter ratio was ranged between 3.99 and 8.02. In the present case, the diameter ratio of cellulose (diameter 20mm) and lignin (diameter 3mm) was 6.67. Based on the above report [22], it was considered that the minimization of void fraction of cellulose and lignin was at 20~30% of mixing ratio of lignin to cellulose. Seeing Figure 2, a WTB using lignin and cellulose was formable in the mixing ratio of 5~30% of lignin to cellulose (dotted area) [13]. Even in the case using cellulose-g-PLLA, the WTB-formal area was limited in the dotted area although PLLA chain might act as an obstacle for a packing.
Figure 5: Possible mechanisms of WTB formation. The softening temperatures for cellulose and lignin are 240 °C and 60 °C, respectively. This sequential process is driven by the pressurized condition (25MPa) for 10min.
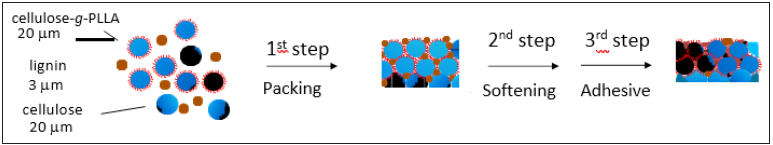
Thus, the first step of the WTB formation could be explained based on the void fraction-dependent packing model of particles with two different diameters. Next, a second step is discussed. The softening temperature of cellulose and lignin in the range of more than 30% of water content is 240 °C [21] and 60 °C [14], respectively. The formable region of WTBs ranged between 40 and 50% of water content as shown in Figure 2, implying the possible contribution of water-assisted softening of cellulose or lignin to the WTB formation. The temperature condition of the present preparation method of WTBs was 180 °C, resulting in the softening of only lignin. Once lignin is softened under the hydrothermal condition, the subsequent cooling of lignin would be relaxed from the softening-state associated with the progression of the hydrogen bonding network not only in lignin-lignin but also lignin-cellulose. An excess or deficiency of lignin would not be expected to have this effect as stated in ref. [13]. Such a process might contribute to the adhesive process as a third step. In the field of crystallography and solid-state physics, an in continuous boundary surface of crystals remained between one and other crystals is termed the grain boundary. It has been well-known, from XRD study, that cellulose particle has the high crystallinity [13,23-26]. It would be then plausible that crystalline cellulose has the grain boundary between cellulose particles through a compression molding. Since the average diameter of lignin (3mm) was herein smaller than that of cellulose (20mm), lignin particles were easy to exist in the grain boundary. FTIR and dielectric studies also demonstrated that lignin particles favored the surface of WTBs [13]. It is, therefore, considered that lignin softened not only in the grain boundary of cellulose particles but also on the surface of assemblies of particles might play a role for the stable formation of WTBs. A use of cellulose-g-PLLA would alter the state of the grain boundary in WTBs. In actual, the narrowed region of WTB formation was observed as shown in Figures 2a & 2b. The PLLA layer on cellulose particles might act as steric effect, i.e. the obstacle to the access of lignin particle. Another role of PLLA layer on cellulose particles was discussed to clarify the role of lignin in the formation of WTBs. In the field of glue or adhesive, an entanglement between polymer chains has the critical role for the adhesive between interfaces [19]. If the entanglement of depolymerized cellulose is a main contribution to the formation of WTBs, the alteration of entire cellulose by cellulose-g-PLLA should enhance the adhesive force between particles. Meanwhile, Figure 3b demonstrated the finite effect of PLLA to the formation propensity of WTBs. Keeping in mind that the use of cellulose-g- PLLA elevated the contact angle (Figure 3), it was plausible that cellulose-g-PLLA might distribute to the surface of WTBs. This means that homo particle (cellulose particle) pushed out the hetero particles (cellulose-g-PLLA) during the process of compression molding, as schematically shown in Figure 3b. This might be a kind of monotectoid although the further investigations regarding the packing of homo and hetero particles under the compression molding would be required. PLLA chains existed in grain boundary can entangle with other PLLA because the molecular entanglement weight of PLLA was 9,000 [27] that was lower than the molecular weight of modified PLLA (Mw=10,000). This effect was associated with the glue-like behavior of lignin particles in grain boundary or the surface, possibly resulted in the long-term stability of WTBs (Figure 4b).
Conclusion
Further improved effect on water repellency was attempted by the chemical modification using cellulose-g-PLLA synthesized in this study. Lignin and water were mixed with the mixture of cellulose with cellulose-g-PLLA to prepare chemically modified WTBs. The formable region of WTBs was limited similar to the bare WTB [13]. The best performance of water repellency for WTBs prepared by cellulose-g-PLLA was 126deg of the contact angle, as compared with 50deg for bare WTB. The present method indicated the higher contact angle than the dipping methods. In addition, the long-term stability (90% in six years) was observed. Thus, these results demonstrated the significance of the chemical modification of cellulose using polymer. The present finding would give a better insight on the modification of the hydrophobic polymer on the surface of cellulose particles to improve the water repellency of WTBs. Further investigations from the viewpoints of monotectoid phenomena would give deeper understanding not only of the effect of chemical modification for WTBs but also the entanglement of polymers. Recently, graft polymerization of cellulose or cellulose derivatives has been developed [28-31]. The present finding would give new extension of green chemistry and sustainable development using these graft polymers based on cellulose.
Acknowledgements
This research was also supported by Yakumo Foundation (2016) about the preparation of PLLA. This work was no conflict of interest to declare.
References
- Nagamatsu Y, Funakoshi M (2003) Design of recyclable matrixes from lignin-based polymers. Green Chem 5: 595-601.
- Almaa MH, Maldasb D, Hafizou H (1995) Water repellency of several wood species impregnated with vinyl monomers. Int l J Polym Mat Polym Biomat 30: 159-165.
- Ayrilmis N (2011) Effect of fire retardants on surface roughness and wettability of wood plastic composites panels. BioResources 6(3): 3178-3187.
- Ozdemir T, Mengeloglu F (2008) Some properties of composite panels made from wood flour and recycled polyethylene. Int J Mol Sci 9(12): 2559-2569.
- Giri CP, Jha A, Chandrakar O (2013) Breaking load analysis of wood plastic composite materials with surface roughness variation. J Mechan Civil Eng 5(6): 29-32.
- Afrifah KA, Hickok RA, Matuana LM (2010) Polybutene as a matrix for wood plastic composites. Compos Sci Technol 70: 167-172.
- Lei Y, Wu Q (2010) Wood plastic composites based on microfibrillar blends of high density polyethylene/poly(ethylene terephthalate). Bioresour Technol 101(10): 3665-3671.
- Leu SY, Yang TH, Lo SF, Yang TH (2012) Optimized material composition to improve the physical and mechanical properties of extruded wood-plastic composites (WPCs). Construt Buil Mat 29: 120-127.
- Pendleton DE, Hoffard TA, Adcock T, Woodward B, Wolcott MP (2002) Durability of an extruded HDPE/wood composite. Forest Prod J 52(6): 21-27.
- Yeh SK, Agarwal S, Gupta RK (2009) Wood-plastic composites formulated with virgin and recycled ABS. Compos Sci Technol 69(13): 2225-2230.
- Mwalkambo L, Ansell MP (1999) The effect of chemical treatment on the properties of hemp, sisal, jute and kapok for composite reinforcement. Die Angew Makromol Chem 272(1): 108-116.
- Bisanda E, Ansell MP (1991) The effect of silane treatment on the mechanical and physical properties of sisal-epoxy composites. Compos Sci Technol 41(2): 165-178.
- Shimanouchi T, Kamba T, Yang W, Aoyagi S, Kimura Y (2015) Surface properties of woody thin boards composed of commercially available lignin and cellulose: Relationship between the orientation of lignin and water repellency. Appl Surf Sci 347: 406-413.
- Eriksson I, Haglind I, Lindbrandt O, Sahnen L (1991) Fiber swelling favoured by lignin softening. Wood Sci Technol 25: 135-144.
- Ma M, Hill RM (2006) Superhydrophobic surfaces. Current Opinion in Colloid & Interface Science 11(4): 193-202.
- Hsieh CT, Chang BS, Lin JY (2011) Improvement of water and oil repellency on wood substrates by using fluorinated silica nanocoating. Appl Surf Sci 257(18): 7997-8002.
- Kadokawa J, Murakami M, Kaneko Y (2008) A facile method for preparation of composites composed on cellulose and a polystylene-type polymeric ionic liquid using a polymerizable ionic liquid. Compos Sci Technol 68: 493-498.
- Dong H, Xu Q, Li Y, Mo S, Cai S, et al. (2008) The synthesis of biodegradable graft copolymer cellulose-graft-poly(L-lactide) and the study of its controlled drug release. Colloids Surf B Biointerfaces 66(1): 26-33.
- Cole PJ, Cook RF, Macosko CW (2003) Adhesion between immiscible polymers correlated with interfacial entanglements. Macromolecules 36(8): 2808-2815.
- Rodrigues AKO, Maia DLH, Fernandes FAN (2015) Production of lactic acid from glycerol by applying an alkaline hydrothermal process using homogeneous catalysts and high glycerol concentration. Brazilian Journal of Chemical Engineering 32(3): 749-755.
- Szczesniak L, Rachocki A, Tritt GJ (2008) Glass transition temperature and thermal decomposition of cellulose powder. Cellulose 15: 445-451.
- Suzuki M, Yagi A, Watanabe T, Oshima T (1984) Estimation of void fraction in a three component random mixture of spheres. Kagakukogaku Ronbunshu 10(6): 721-727.
- Park S, Baker JO, Himmel ME, Parilla PA, Johnson DK (2010) Cellulose crystallinity index: measurement techniques and their impact on the interpreting cellulose performance. Biotechnol Biofuels 3: 10.
- Baker AA, Helbert W, Sugiyama J, Miles MJ (1997) High-resolution atomic force microscopy of native valonia cellulose I microcrystals. J Struct Biol 119(2): 129-138.
- Iwamoto S, Kai W, Isogai A, Iwata T (2009) Elastic modulus of single cellulose microfibrils from tunicate measured by atomic force microscopy. Biomacromolcules 10(9): 2571-2576.
- Ishikawa A, Okano T, Sugiyama J (1997) Fine structure and tensile properties of ramie fibers in the crystalline form of cellulose I, II, IIII and IVI. Polymer 38(2): 463-468.
- Dorgan JR, Williams JS (1999) Melt rheology of poly(lactic acid): Entanglement and chain architecture effects. J Rheol 43(5): 1141-1155.
- Guo Y, Liu Q, Chen H, Wang X, Shen Z, et al. (2013) Direct grafting modification of pulp in ionic liquids and self-assembly behavior of the graft copolymers. Cellulose 20(2): 873-884.
- Zhang L, Guo Y, Zhou J, Sun G, Han Y, et al. (2016) Synthesis and characterization of cellulose-graft-poly(p-dioxanone) copolymers via homogeneous ring-opening graft polymerization in ionic liquids. BioResources 11(1): 2698-2711.
- Ge W, Guo Y, Zhong H, Wang X, Sun R (2015) Synthesis, characterization, and micellar behaviors of hydroxyethyl cellulose-graft-poly(lactide/ε-caprolactone/p-dioxanone). Cellulose 22(4): 2365-2374.
- Guo Y, Wang X, Shen Z, Shu X, Sun R (2013) Preparation of cellulose-graft-poly(ε-caprolactone) nanomicelles by homogeneous ROP in ionic liquid. Carbohyd Polym 92(1): 77-83.
© 2021 Yukitaka Kimura. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






