- Submissions

Full Text
Polymer Science: Peer Review Journal
Preparation of Ionic Liquid Crystals and their Influence in TPU/SAN Melt Blends as Compatibilizers for Displays
Sellamuthu N Jaisankar1,2*, Mir Muhammad Fahad1, Mohammad Shamim Reza1, Gajula Prasad1, Kap Jin Kim1 and Hongdoo Kim1*
1Department of Advanced Materials Engineering for Information and Electronics, Kyung Hee University, Korea
2Polymer Science & Technology Division, CSIR-Central Leather Research Institute, India
*Corresponding author: Sellamuthu N Jaisankar and Hongdoo Kim, Department of Advanced Materials Engineering for Information and Electronics, Kyung Hee University, Yongin, Gyeonggi-do 17104, Korea
Submission: September 11, 2021;Published: September 24, 2021

ISSN: 2770-6613 Volume2 Issue2
Abstract
The novel synthesis of novel Ionic Liquid Crystal Polyurethanes (ILCPUs) (main-chain liquid crystals with the ionic group as a side group) was successfully demonstrated. Sulfonic acids were used as negative group and sodium ions as a positive charge. All the synthesized compounds show low molecular weight ILCPUs and exhibit nematic phase is a very rare mesophase for ionic liquid crystals. The melt compatibilization of Thermoplastic Polyurethane (TPU) and Styrene-Acrylonitrile (SAN) blends by Ionic Liquid Crystalline Polyurethanes (ILCPUs) were prepared using a two-roll mill at 220-235 °C. The results obtained from the various analyses suggest that ILCPUs could be used as an effective compatibilizer for the immiscible polymers blends.
Keywords: Two-roll mill; Melt blend; Rheology; Activation energy; Liquid crystals
Abbreviations: ILCPUs: Ionic Liquid Crystal Polyurethanes; TPU: Thermoplastic Polyurethane; SAN: Styrene-Acrylonitrile; LC: Liquid Crystal; DMA: Dynamic Mechanical Analyses
Introduction
Melt mixing of two polymers results in blends, which may be miscible or immiscible depending on the interaction parameter of the constituent polymers. In the case where the melt blending leads to immiscibility, and in-situ chemical interaction such as hydrogen bonding enhances phase mixing thereby imparting miscibility [1]. For the past two decades, many authors have tried to exploit the superior properties of thermotropic liquid crystals by blending them with thermoplastic polymers. Several reviews summarize the published literature on this subject [2,3]. In the recent years, the researchers have focused on developing methods for improving the compatibility of liquid crystals and other thermoplastics. The interfacial adhesion between immiscible polymers may be improved either by adding a third, interfacial active martial called compatibilizer or by promoting chemical reaction between the two polymers that effectively form graft copolymers in situ that serves as the compatibilizers. An example of physical compatibilizers i.e. non-reactive compatibilization, for LC blends are not effective for the display applications. It has been shown that ionic clustering and physical gelation is compatible with LC phase [4]. The completion between the formation of ionic clusters and LC phase depends on the chemical structure, which is stronger in the LC mainchain ionomers than LC side chain ionomers. The Liquid Crystal (LC) ionomers were used as compatibilizers that can explain the influences of the anisotropic charge distribution on the mesophase stability of ionic liquid crystals. The important reason for studying such a system is because the incorporation of the metal ion in an ordered polymer matrix opens a way to the novel generation of functional materials with a set of valuable properties. In the liquid crystal ionomers, the metal atoms play the role of counter ions, which compensate for the negative charge of the functional fragments bonded to the polymer chain. One of the important classes of the LC ionomers combines both liquid crystal ionomeric polymers and amorphous polymers. This could be explained in the following way: an isodiametric mesogenic groups of LC ionomers can form various LC phases like nematic, cholesteric (Chiral Nematic) and smectic. Generally, all the ionomers will exhibit the smectic liquid crystalline phase. At the same time, their charged groups can form ion aggregates (multiples, clusters) that act as points of noncovalent polymer chain cross-links and specific morphology and properties of the usual statistical ionomers. The main factors that control the phase behaviour and structure of liquid crystal ionomers are the concentration of the metal ions (<10%), the polymer matrix, and structure [5-7].
The mechanical and structural properties of the blends mainly depend upon the content and morphology of LCPs. It is known that the nematic LCPs can be oriented parallel to the direction of the flow in the liquid crystalline states. This feature of the LCPs is almost preserved also in the blended melt containing conventional thermoplastic polymers. Therefore, LCPs are believed to play a role as processing aid and reinforcement of matrix [8]. Moreover, to our best knowledge, no paper has been reported on the systematic investigation on compatibilization of the TPU/ SAN blends by functionalized and ionomeric liquid crystals. The present investigation deals with the blends of Thermoplastic Polyurethane (TPU) and Styrene-Acrylonitrile (SAN) with or without compatibilizer blended by melt mixing in a two-roll mill. Different compatibilizers based on novel functionalized and ionomer liquid crystals were prepared, and their liquid crystalline phase was identified and incorporated in the TPU/SAN melt blends and their influence of morphological, dynamic and mechanical properties were studied.
Experimental
Materials
Thermoplastic Polyurethane (TPU), Desmopan 385 was obtained from Bayer–Chemplast. Styrene Acrylonitrile (SAN) copolymer with acrylonitrile content 24% commercial-grade (ABSOLAN 2300) was obtained from ABS industries. Thermotropic Liquid Crystalline Polyurethane (ILCPUs) was synthesized using 4,4’-cyanohydroxybiphenyl as shown in Scheme 1. Dihydroxybenzoic acid, dihydroxypropionic acid, 6-chloro-1- hexanol, 4,4΄-Dihydroxybiphenyl, Dibutyltin Dilaurate (DBTDL), 4΄-Hydroxy-4-Biphenylcarbonitrile, 1,6-Diisocyanato Hexane (HMDI), Isophorone Diisocyanate (IPDI), 4,4΄-Methylene Bis (Phenyl Isocyanate) (MDI), Tolylene-2,4-Diisocyanate (TDI) (Aldrich, USA). N, N΄-Dimethylformamide (DMF), Tetrahydrofuran (THF), methanol, methyl pyrrolidone, butyl alcohol, petroleum ether (60-80 ˚C), Aldrich. The solvents used were of analytical grade and purified and dried as per the standard procedures.
Preparation of ionic liquid crystalline polyurethanes
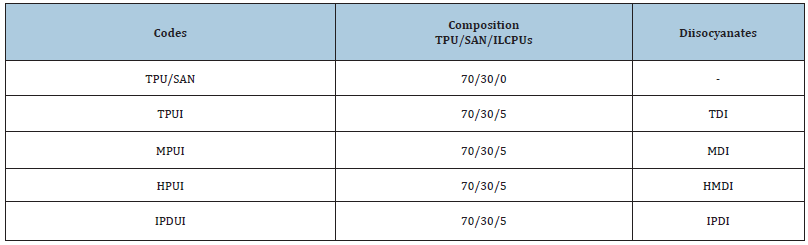
4-4’-bis (n–Hydroxyalkyaloxy) Biphenyl (HHBP) was synthesized according to the method described in the literature. HHBP was dissolved in DMF and heated up to 50 ˚C. An excess of isocyanates (TDI, HMDI, MDI and IPDI) was added slowly to yield -NCO terminated prepolymers. When the NCO content of 5.5- 2.5% was reached as determined by the n-dibutyl amine method, the calculated amount of ionic diols in DMF was added slowly to the prepolymer at 50 ˚C. The reaction temperature was raised to 70 ˚C and continued till the NCO groups reacted as confirmed by the disappearance of the IR absorption of NCO at 2270cm-1. Quaternization of the SO3H groups in DMF was carried out by the addition of different metal oxide/acetates (Scheme 1). The mixture was poured into cold methanol and the product was filtered and reprecipitated. TPU and SAN were dried at 80 ºC for 24 hours. These materials were blended in different weight ratios (100/0, 90/10, 70/30, 50/50, 30/70 and 0/100) in a two-roll mill at 220-235 ºC at milling time 10-12 minutes. These blends were compression moulded in between two plates kept at 200-210 ºC with a load of 10MPa to get sheets of 250x50x5mm. The blend 70/30 ratio was selected based on thermal, mechanical and rheological studies and blended with different compatibilizers of ILCPUs in a two-roll with the same temperature and milling time. The codes and composition of the compatibilized blends are given in Table 1.
Scheme 1:Preparation of ionic liquid crystals.
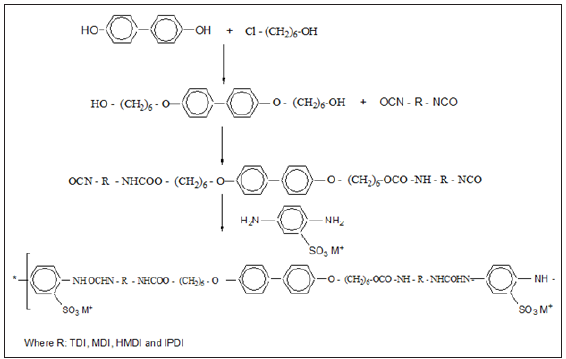
Table 1: Codes and Compositions of the TPU/SAN/ILCPUs blends.
Dynamic Mechanical Analyses (DMA) was determined in a dynamic mechanical analyzer DMA-TA Instrument 2980 to measure the dynamic viscoelasticity and temperature performance of polymers. Compression moulded samples of the size 50mmx4mmx0.5mm were used for this purpose. The test was carried out at a heating rate of 5 ºC/min and a frequency of 10Hz at temperatures ranging from–60 to 130 ºC. The tan δ (loss tangent or damping factor), storage modulus (E’) and loss modulus (E’’) were read directly from the instrument. Hot Stage-Polarized Optical Microscope (HOPM) optical micrographs were taken with a Reichert–Jung Thermogallen hot stage optical microscope with a magnification of 100X (10x10 Bausch & Lomb eyepiece and lens), attached with a polarizer and an Asahi Pentax 35mm camera with Pentax bayonet mount and 25mm F eyepiece tube. Photographs were taken at ½ seconds shutter speed using 35mm 125 ASA / ISO color film just before the clearing of the liquid crystal polyurethane phase. FTIR analyses were done with a Nicolet impact 400 FTIR Spectrophotometer to identify the functional groups and the nature of specific interactions involved between the polymers. Cone and plate Rheometer (Rheometer AR 500, TA Instruments) was used to measure the melt viscosity (η), as a function of the shear mode. The diameter of the plate was 25mm, and the cone angle was 4º. Data acquisition was accomplished with the aid of a microcomputer interfaced with rheometer. Rheological measurements were made at different temperatures for the TPU/SAN blends and ILCPUs compatibilized blends in the angular frequency range from 0.1 to 100rad/s. The temperature control was satisfactory within +1ºC.
Results and Discussion
Infrared spectroscopy is a powerful technique for the investigation of hydrogen bonding in carbonyl compounds. The urethane compound based on HMDI, an aliphatic diisocyanate shows a fairly sharp peak with maxima at 1685cm-1, which also has a distinct shoulder at 1695cm-1. This shoulder is most probably due to the presence of several isomers in HMDI such as trans, trans (E,E), trans, cis (E,Z) and cis,cis (Z,Z). The typical IR spectrum of the samples showed the bands near 3330cm-1 (N-H stretching), 1700cm-1 (C=O stretching), 1540cm-1 (C-N-H bending) and 1280 cm-1 (N-C-O stretching). The formation of the low molecular weight compound polyurethane ionomers confirmed by the disappearance of OH stretch (broad peak) at 3350-3535cm-1, and the appearance of a new sharp peak for N-H stretching 3330cm-1 and for NHCOO (urethane) at 1651 cm-1 verify the formation of polyurethane. The FTIR spectra of ionic liquid crystal polyurethanes of TPUI, HPUI, MPUI and IPUI confirm the presence of NH group at 3332-3342cm- 1 and aromatic and aliphatic peaks at 2933-2941 cm-1 and 2857- 2868cm-1. The presence of ionic groups at 1246-1256 cm-1 and 822- 834cm-1 confirm the formation of ionic polyurethanes.
Hot stage polarizing optical microscope (HPOM)
The texture of ionic liquid crystals was observed by Polarizing Optical Microscope (HPOM) with a hot stage as shown in Figure 1. The phase behaviour of liquid crystals mainly depends on the nature of the polymer backbone, the rigidity of the mesogenic unit, and the length of the flexible spacer. HPOM observation results showed that TPUI exhibited enantiotropic schlieren double axial phase and HPUI revealed nematic phase on cooling cycles. TPUI was heated to 208 ˚C, the sample began to melt, and exhibit schlieren double axial texture retained up to 264 ˚C, and then reached the isotropic state. While cooling, the schlieren double axial texture appeared at 136.9 ˚C. If a mechanical field were superimposed on the sample, for example, slight shearing of the melt would cause the macroscopic orientation of the double axial domains, which is a typical characteristic of functionalized LCs The MPUI sample began to melt at 254 ˚C and reached the isotropic state at 300 ˚C. The sample was cooled from an isotropic state and shows nematic texture. Photomicrograph of IPDI is shown in Figures 1a & 1b, the optical textures of ionomers are typical nematic textures. All the polymers exhibited thermotropic LC properties during heating and cooling. For LC ionomers, it has been shown that ionic clusters are compatible with the LC phase. Competition between the formation of ionic cluster phases and the LC phase depends on the chemical structure. Because there were few ionic groups in the LCP systems, the ionic aggregation would have been tangled with the rigid mesogenic groups of LC segments to form multiple block domains. The ions aggregated in the domains due to their electrostatic interactions, thus forcing the soft main chains to fold and form a structure. These illustrated the appearance of the nematic mesophase for polymers of TPUI, HPUI, MPUI and IPUI (Table 1) with the ionomeric content (<10%) in the LCP systems (Scheme 1).
Figure 1a: Cross polarized optical micrograph of IPDI, at T=136.9 ºC (cooling, 100X).

Figure 1b: Photomicrographs (cooling, liquid crystal texture 100X): HMDI at T=110.3 ºC

TPU/SAN melt blends
The pure TPU was characterized by a sharp peak at 1732cm- 1 due to carbonyl group stretching vibration and N-H stretching frequency of urethane peaks at 3335cm-1. In general, four peaks in the carbonyl, region would be expected for polyester polyurethane i.e., free and bonded peaks of carbonyl groups. The experimental results implied a close overlap of these bonds. The FTIR spectrum of TPU/SAN showed a decrease in the intensity of the hydrogenbonded carbonyl group in the TPU matrix with an increase in the acrylonitrile content. Miscibility of polymer blends can be obtained from the viscoelastic data obtained by Dynamic Mechanical Analysis (DMA). From the DMA thermogram, the storage modulus (E’) of the SAN was higher than that of the TPU. The a- relaxation temperature of the SAN at about 101 °C appears to be associated with the onset of cooperative motions of SAN chain. The storage modulus of the blends at 30 °C increased with increasing SAN content. It was found that the SAN reinforced the TPU matrix, and the storage modulus decreased very slowly with increasing temperature. The storage moduli of blends have an intermediate value of SAN and TPU at all compositions. The results imply that the TPU exhibits better compatibility with SAN, in the 70/30 blend ratios and a proportional decrease in the E’ is observed. While in TPU/ SAN 70:30 blends, E’ decreases after Tg. The results indicate that the microphase separation of the hard segments takes place and provide self-reinforcements of the TPU, leading to high modulus. The tan d of SAN is shown as a maximum at around 101 °C. This is believed to be a relaxation for the SAN at this temperature. On increasing the TPU ratio in the blend the Tg decreases. The melt viscosity of TPU, SAN and their blends were recorded against shear rate. The effect of temperature on the melt viscosity of TPU/ SAN blend systems are presented in Table 2. The thermodynamic activation parameters from melt viscosity measurements using the following Eyring equation [10].
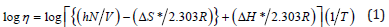
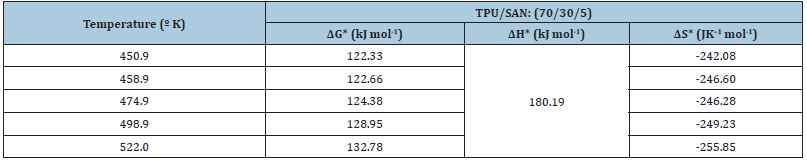
Table 2: Thermodynamic activation parameters calculated from rheometric data for TPU/SAN (70/30) blends.

where η: dynamic viscosity; h: Plank’s constant; N: Avogadro’s number; V: molar volume of the solvent; R: universal gas constant; T: absolute temperature. The enthalpy of activation (ΔH) values were calculated from the slope [ln(1-C) vs 1/T]. The Gibbs’s free energy of activation (ΔG) was calculated using the following equation
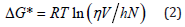
The entropy of activation DS* has been calculated using the following Gibbs’s equation
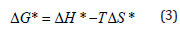
The thermodynamic activation parameters calculated from the log η versus (1000/T) graph for TPU/SAN blends system is presented in Table 2. It can be seen from the table that over the entire range of investigated temperatures, Gibb’s free energy of activation ΔG* increases with the increase in temperature. The increase of ΔG* clearly indicates that the work done by the system increases with an increase in temperature ΔH* and DS* values were lower in the case of blends when compared to the pure TPU. The results suggest that thermal stability of the blends are higher [9]. In this study, the liquid crystal ionomer (LC) compatibilizers were liquid crystalline polyurethanes TPUI, MPUI, HMPUI, HPUI and the matrix used was TPU/SAN (70:30). The structure of the blends was characterized by the FTIR spectrophotometer. The peak at 2275cm-1 is the characteristic stretching peak of –CN for SAN; the peak intensity of which increases with the addition of different compatibilizers. The viscoelastic properties of polymers depend on the thermal history of samples because it influences their morphology. In (Figure 2), tan δ in a temperature range from –70 ºC up to 85 ºC is presented. From (Figure 2), two-loss peaks are observed for the compatibilized blends. The first one appears at temperature -30.2 ºC, - 26.3 ºC, 13.87 ºC, and –32.83 ºC for the sample blends of HPUI, MPUI, HMPUI and TPUI respectively. The intensity and the location of this peak are strongly dependent on the chemical composition of the samples. By increasing the aromatic urethane content (hard segment content of TDI and MDI) these motions are restricted, shifting the transition temperature to higher temperature. The tan δ of the TPU/SAN components in the blend was also shifted. This means that the ILCPUs are compatible with the TPU/SAN matrix.
Figure 2:Tan δ against Temperature for TPU/SAN blend with different compatibilizers.
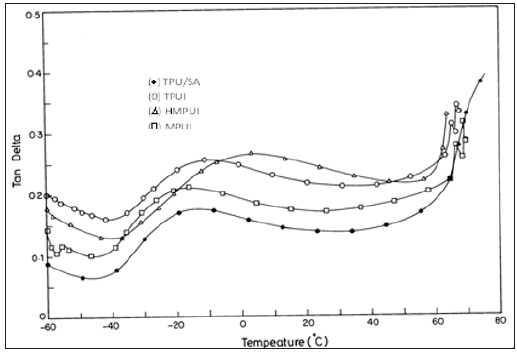
Figure 2 shows a plot of tan δ as a function of temperature for the TPU/SAN and the compatibilized blends. The tan δ of TPU/ SAN blend showed maximum around 69.49 °C. This is believed to be due to the α relaxation of the TPU/SAN blend. In the same temperature region the storage modulus of the blends decreases by approximately two orders of magnitude, especially at the vicinity of the glass transition temperature (Tg) of the SAN as seen in Figure 1. (Figure 3), DMA results show the loss modulus (E’’) as a function of temperature for the blends. The loss modulus and storage modulus of the blends, decreased very slowly with increasing temperature (Table 3). It is presumed that the chain alignment is still maintained in the LCPs. The HPU and HMPU are better compatibilizers than TPUI and MPU. This is inferred from the tan δ values given in Table 3. The tan δ for the compatibilizer blends are 0.46, 0.47 and 0.58. For the blend compatibilized with HPU, tan δ values are between 0.1-0.5 indicates compatibility. The hard segment in HPU does its role as compatibilizer/ physical cross-linker site or reinforcing filler more effectively. The rheological measurements were performed with the cone and plate rheometer. All the melts exhibited non- Newtonian flow behavior in the shear rate range studied [10]. The thermodynamic activation parameters for TPU/SAN/ILCPU (HMDI) compatibilized blends were calculated from log η versus (1/T) graph as shown in Table 4. The viscosity of the compatibilized blends did not show shear-thinning behavior, which could be due to the small addition of LCP. As the temperature increased, ΔH* and DS* values were lower in the case of compatibilized blends when compared to the TPU/ SAN. The results suggest that the thermal stability of the blend is higher.
Figure 3:Loss modulus against Temperature for TPU/SAN blend with different compatibilizers.
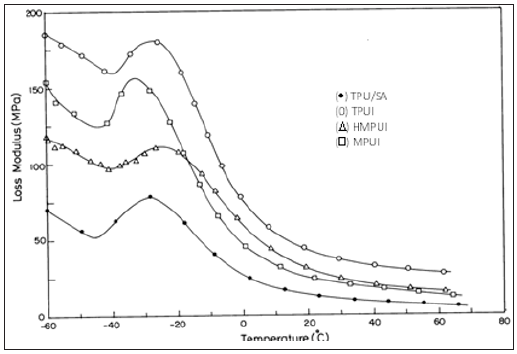
Table 3: DMA data for TPU/SAN/TPUI compatible blends.
Table 4:Thermodynamic activation parameters calculated from rheometric data for TPU/SAN/TPUI (70/30/ 5) compatibilized blends.
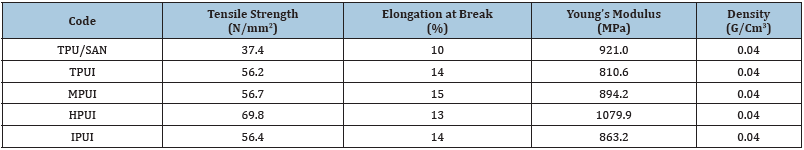
The stress-strain properties of ILCPUs are associated with the formation of a fibrillar structure. Chang et al. [11,12] have reported that the addition of LCP to the polymer matrix caused a significant increase in tensile properties [13-16]. The strength of the blend containing as low as 1% LCP was higher than that of polymer. It appears that there is a critical amount of LCP beyond which the liquid crystalline polymers reinforcing capability diminishes. Based on the above we have selected 5% of different LCPs, which were added into the TPU/SAN blend matrix. Table 5 shows the value of the tensile modulus and tensile strength of all the ILCPUs. Tensile strength is increased by 10% when HPU based TLCP is incorporated into the TPU/SAN matrix. The same behavior is observed for the tensile modulus. In all cases, increases in elongation at the break did not exceed 5%.
Table 5:Physical properties of TPU/SAN/TPUI compatible blends.
Conclusion
We have shown that all the synthesized ionic liquid crystals show the nematic mesophase. The melt blends of TPU/SAN copolymer of various compositions were prepared using a two-roll mill. DMA and mechanical properties reveals that the TPU/SAN blends are miscible to a very limited extent. All the compositions of TPU/SAN blends studied only 70:30 blend ratio showed slightly better results hence, this blend was chosen for compatibilization studies. The use of low molecular weight ILCPUs, showed good compatibilizers for TPU/SAN melt blends.
Acknowledgment
This work was supported under the Brain Pool Program by the National Research Foundation of Korea (Grant #: 2020H1D3A2A01103375). S. N. Jaisankar, thanks to CSIR-CLRI for sabbatical leave support while performing this work.
References
- Utracki LA (1989) Polymer alloys and blends, Hanser Pub Munich, Vienna, New York.
- Dutta D, Fruitwala H, Kohli A, Weiss RA (1990) Polymer blends containing liquid crystals: A Review. Polym Eng Sci 30(17): 1005-1018.
- Weiss RA, Ghebremeskel Y, Charbonneau L (2000) Miscible blends of a thermotropic liquid crystalline polymer and sulfonated polystyrene ionomers. Polymer 41(9): 3471-3477.
- Prasad KS, Baral M, Murali A, Jaisankar SN (2017) Carbon nanotube reinforced polymer-stabilized liquid crystal device: lowered and thermally invariant threshold with accelerated dynamics. ACS Applied Materials & Interfaces 9(31): 26622-26629.
- Nikonorova NA, Borisova TI, Barmatov EB, Apebalk D, Calleja RD (2004) Molecular mobility of CU(II)-containing liquid crystalline ionomers: dielectric relaxation and thermally stimulated depolarization currents. Polymer 45(5): 1555-1562.
- Datta A, Baird DG (1995) Compatibilization of thermoplastic composites based on blends of polypropylene with two liquid crystalline polymers. Polymer 36(3): 505-514.
- Tjong SC, Xu SA, Mai YW (2003) Impact fracture toughness of short glass fiber-reinforced polyamide 6,6 hybrid composites containing elastomer particles using essential work of fracture concept. Mate Sci and Eng 374(1-2): 338-345.
- Balakrishnan S, Neelakantan NR, Jaisankar SN (1999) Effect of functionality levels and compatibility of polycarbonate blends with maleic anhydride grafted ABS. J Appl Polym Sci 74(8): 2102-2110.
- Jaisankar SN, Radhakrishnan G (2000) Effect of compatibilizer on morphology and mechanical properties of TPU/SAN blends. Polym Eng Sci 40(3): 621-626.
- Mandal AB, Subramanian V, Somanathan N (1997) Thermal stability of modified caseins. Thermochimica Acta 302(1-2): 47-52.
- Chang JH, Jo BW, Jin JI (1995) Transesterifications in a polyblend of poly(butylene terephthalate) and a liquid crystalline polyester. Polym Eng Sci 35(20): 1615-1620.
- Yue H, Al-Shujaa SAS, Zhen B, Zhang Y, Li X, et al. (2021) Blue nanocomposites coated with an ionic liquid polymer for electrophoretic displays. RSC Advances 11: 20760-20768.
- Tjong SC, Joang W (2000) In-situ reinforcement of PBT/ABS blends with liquid crystalline polymer. Polymer Composites 21(6): 941-952.
- Roeder J, Oliveira RVB, Becker D, Gonçalves MW, Soldi V, et al. (2005) Compatibility effect on the thermal degradation behaviour of polypropylene blends with polyamide 6, ethylene propylene diene copolymer and polyurethane. Polymer Degradation and Stability 90(3): 481-487.
- Binnemans K (2005) Ionic liquid crystals. Chem Rev 105(11): 4148-4204.
- Dhevi DM, Prabu AA, Kim KJ (2016) FTIR studies on polymorphic control of PVDF ultrathin films by heat-controlled spin coater. J Mater Sci 51: 3619-3627.
© 2021 Sellamuthu N Jaisankar and Hongdoo Kim. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






