- Submissions

Full Text
Polymer Science: Peer Review Journal
Quo Vadis Progress in Polymer Stabilization?
Jiri Tochacek*
CEITEC-Central European Institute of Technology, Brno University of Technology, Czech Republic
*Corresponding author: Jiri Tochacek, CEITEC-Central European Institute of Technology, Advanced Polymers and Composites, Brno University of Technology, Purkynova 656/123, 612 00 Brno, Czech Republic
Submission: July 21, 2021;Published: July 28, 2021

ISSN: 2770-6613 Volume2 Issue2
Abstract
Synthetic polymers processed and applied in practice undergo degradation changes that result in the potential loss of physico-chemical properties. Polymer stabilizers are used to prevent these changes and help the polymer utility properties get retained. Despite the visible progress in other fields of human activity, polymer stabilization chemistry has exhibited only minimum changes. No new chemistry of polymer stabilization has been introduced within the several past decades. All the “new” stabilizers promoted by different suppliers were mostly the modifications of the existing ones. Any new and commercially really successful stabilizer capable of competing the performance of old existing structures has not appeared on the market for many years.
Opinion
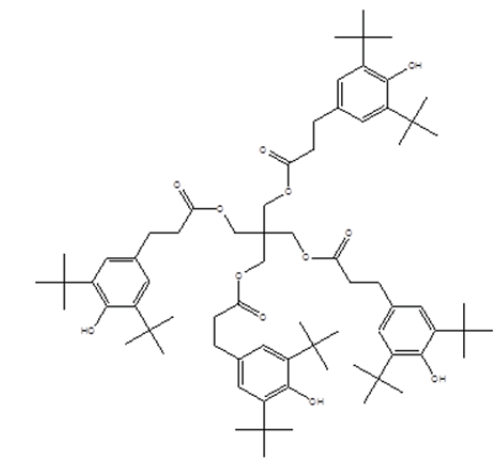
Most of commodity plastics being in contact with natural environment undergo irreversible changes consequently affecting their utility properties. Thermoplastics, such as e.g. polyolefins, are sensitive to oxidation both during processing and end application. They have to be protected by the addition of stabilizers–organic compounds having suitable functionalities capable of chemical reactions competing the degradation processes. The history of polymer stabilization more or less reflects the history of commodity plastics themselves. The commercial development of plastics was closely followed by the development of appropriate stabilizing structures that started in the sixties of the last century. In the sixties and later in the seventies most of the stabilizing structures like sterically hindered phenols, organic phosphites and phosphonates, thio-stabilizers and sterically hindered amines (HALS) were introduced on the market [1-3]. These structure types were found efficient and acceptable in terms of price-performance ratio so that they were widely used in the protection of most of the synthetic polymers. The same structural functionalities either unchanged or slightly modified have been used up to now and during the past fifty years practically no new chemistries appeared. The only exception were lactones launched in the middle of the nineties, at that time considered as a breakthrough in polymer stabilization chemistry [4,5]. Their success, however, dropped shortly after since their active stabilizing moiety was found to be suspect mutageneous so that they were phased out soon after. Since the seventies no real brand-new structures providing a new mechanism of action have appeared. All the progress in polymer stabilization has taken place almost exclusively in the field of modification of existing active functionalities or their secondary structures. Among the modified ones, only few reached commercial success, such as e.g. NOR structures of Hindered Amine Light Stabilizers (HALS) [6]. The rest did not fully meet the toxicological requirements or simply was not efficient enough. Despite in other sectors of polymer chemistry continuous progress in properties and characterization proceeds and is visible every year, in polymer stabilization no changes have taken place already for many decades. If the suppliers offer “new” stabilizers, there are mostly only the blends of existing structures, declaring some kind of benefits or synergy, but definitely no new chemistry. Presently, many structures launched to the market more than half of the century ago are still intensively used. And successfully compete the new much “younger” ones due to their good efficiency and extremely favorable price-performance ratio. One of them, pentaerythrityl tetrakis [3-(3,5-di-tert-Bu- 4-hydroxyphenyl)propionate] well known as Irganox 1010 [7] celebrated in 2013 50th anniversary of its existence on the market and still is “going strong”. Still being used all over the world and produced under the variety of commercial names (Figure 1). This structure represents the most successful polymer stabilizer ever, in polymer stabilization having the same name as Elvis Presley in entertainment industry. One of the reasons of its success may likely be the expiration of its original patents that makes it more easily accessible and allows its friendly price to compete the potentially new structures, the development of which requires considerable investments. Despite these facts are reflective, they still do not fully explain the huge success of this structure in polymer protection persisting more than half of the century. And so there are the questions to be answered. What is the real reason of absence of progress in polymer stabilization? [8]. Is it the lack of interest due to low economic motivation caused by demanding toxicological testing necessary for hygienic approval or simply the fact that the old structures are brilliant so far that they still efficiently beat any newly appearing competition?
Figure 1:Irganox 1010-stabilizing structure of hindered phenol type originally introduced by Ciba-Geigy AG in the sixties - CAS No. 6683-19-8.
References
- Gächter R, Müller H (1990) Plastics Additives. (3rd edn), Hanser Publishers, Munich, Vienna,
- Wolf R, Kaul BL (1992) Plastics, Additives. Ullman´s Encyclopedia of Industrial chemistry, VCH Publishers, Germany.
- Allen NS, Edge M (2021) Perspectives on additives for polymers. 1. Aspects of stabilization. J Vinyl Addit Technol 27: 5-27.
- Kröhnke C (1997) A major breakthrough in polymer stabilization. SPE Conference Polyolefins X RETEC, Houston, Texas, USA.
- Pauquet JR (1999) Breakthrough chemistry for processing stabilization of polypropylene. J M S Pure Appl Chem A36(11): 1717-1730.
- https://polymer-additives.specialchem.com/product/a-basf-tinuvin-nor-371
- https://polymer-additives.specialchem.com/product/a-basf-irganox-1010
- Malík J, Kröhnke C (2006) Polymer stabilization: Present status and possible future trends. C R Chimie 9(11-12): 1330-1337.
© 2021 Jiri Tochacek. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






