- Submissions

Full Text
Polymer Science: Peer Review Journal
Three Dimension Functionalized Graphene for Supercapacitor Application
Samui AB*
Institute of Chemical Technology, Nathalal Parekh Marg, India
*Corresponding author: Asit Baran Samui, Institute of Chemical Technology, Nathalal Parekh Marg, Matunga, Mumbai 400019, India
Submission: May 19, 2021;Published: July 02, 2021

ISSN: 2770-6613 Volume2 Issue1
Introduction
With increasing energy demand the energy conversion and storage have become crucial issues in the world. Considering various non-conventional and seasonal energy sources, electrochemical systems for energy conversion and storage are being considered very important. To realize such devices for matching high-performance criteria, suitable materials are required. The charge separation mechanisms, essential for the energy storage operation of electrochemical capacitors, belong to two different classes, such as Electrical Double-Layer (EDL) formation by ion adsorption, or surface oxidation/reduction (pseudo capacitance) [1]. Unlike batteries, they exhibit long cycle life and fast charging/discharging at high current loads, which make them superior to batteries in terms of power densities. The material’s performance in supercapacitors is described by its capacitance. The oxygen- and nitrogen functional groups, usually present in carbonaceous materials can undergo redox reactions, which results in a pseudo capacitance contribution to the EDL capacitance [2]. Graphene, a single layer of carbon atoms with a hexagonal arrangement in a two-dimensional lattice, has high thermal conductivity, high specific surface area, excellent electronic conductivity, and huge theoretical surface area. These properties are ideal for potential applications in supercapacitor electrodes. Nano-sized conductive fillers like graphene nanoplatelets, can create a percolative network within the polymer matrix at a low weight fraction, while the conductive nano inclusions within a polymer matrix can alter the permittivity of the composite systems, which enhances their energy storing capability [3]. Nano inclusions can be considered as a distributed network of nanocapacitors, which can be charged and discharged that is defined as an energy storing process at nanolevel [4]. If the entire theoretical specific surface area of graphene is fully utilized, a theoretical EDL capacitance value of up to 550Fg−1 can be expected [5,6]. However, the high tendency of graphene sheets to restack during application as electrode materials owing to the strong π–π interactions between neighboring sheets leads to significant decrease in the electrochemically active surface area and accessibility of ions during electrochemical activity. Introduction of a pseudocapacitive component as the second phase can do the job of effectively preventing the restacking of graphene together with contribution to the improvement of the specific capacitance by adding pseudo capacitance to the EDL capacitance. Various graphene-based composites can be designed by coupling with pseudocapacitive materials, such as graphene/RuO2, [7] graphene/MnO2, [8] and graphene/ polymer (polyaniline, polypyrrole) [9] and more, which produce higher specific capacitances than that of graphene itself. However, the high cost, poor rate performance, instability etc. remain critical issues. An alternative strategy can be the functionalization of graphene with pseudocapacitive chemical moieties such as carbonyl and hydroxyl groups [10]. For all the graphene-based electrodes the efficiency improvement of EDLC-PC hybridization is a great challenge. The key issue is the efficient combination of maximum electrically conductive channels and stable and effective pseudocapacitive functional groups in the electrode. Thus, it is very much required to precisely control the surface chemistry of materials in graphene family for high performance EC applications [11].
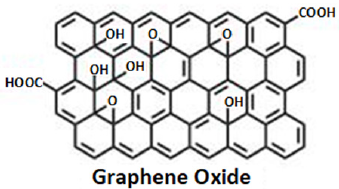
Graphene sheets, prepared by the exfoliation of the graphene oxide formed by chemical oxidization of graphite, contain abundant active sites, such as functional groups (mostly – OH, –COOH, –C=O, C–O–C–, epoxy) (Figure 1). The presence of functional groups on graphene is essential for introduction of active species such as metal oxide, which are targeted to improve the Pseudo Capacitance (PC) together with the total performance of graphene based supercapacitor electrodes. However, poor rate capability due to the large volumetric change or degradation is the characteristics of such hybrids. There are continuous searches for graphene modification towards better performance coupled with stability. Functionalization of graphene is thus one of the key topics in graphene research. The functionalization can be divided in to two main categories: chemical and nonchemical. Chemical functionalization is done via formation of new covalent bonds between the atoms of Reduced Graphene Oxide (RGO)/ Graphene Oxide (GO) and the incoming functional groups. On the other hand, the nonchemical functionalization is mainly based on π interaction between guest molecules and RGO/GO. Although, both types of functionalization can impart some property to graphene, the chemical routes are more effective. Various chemical routes proposed and tried including heterogeneous atoms doping, diazonium coupling, amidation, silanization, esterization, substitution, cycloaddition, etc. The size of graphene is known to affect strongly the reactivity of graphene and the application of the functionalized graphene [12]. Also, the carbon atoms on the ribbon edge are found more reactive than those in the middle of the ribbon. The chemical functionalization alters the electric conductivity of graphene to large extent and introduces some defect sites, which adversely affects the application of the functionalized graphene in energy conversion and storage applications. The heterogeneous atoms doping improves the electric conductivity of graphene and hence, the corresponding functionalized graphene has received more attention [13].
Figure 1: Schematic representation of graphene oxide.
Chemical Functionalization of Graphene
Graphene can be chemically functionalized by two ways. The first process depends on the linking of heterogeneous atoms onto the basal plane forming covalent bond directly with C atoms of graphene. In the second process covalent bonds are established between the functional group present in GO and the guest functional groups. The introduction of heterogeneous atom is relatively easier and also has better electrochemical properties. The bonding of Nitrogen atom improves the electronic conductivity and offers more active sites (defects) [14]. Chemical Vapour Deposition (CVD) is one of the important processes, which can be done by controlling three key components, i.e., catalyst, reactants and high temperature [15]. Nitrogen plasma method can be used to introduce nitrogen on graphene by using in situ Electron Cyclotron Resonance (ECR) plasma at a low-pressure and room-temperature. N+-ion irradiation synthesis involves N+-ions bombardment of graphene followed by annealing of the bombarded graphene in NH3 [16]. Arc discharge method is also attempted by using pyridine, NH3 as the reaction environment along with hydrogen. Electrothermal reaction involves high-power electrical joule heating of Graphene Nano Sheets (GNS) in ammonia gas [17]. N-graphene with high nitrogen level (up to 10 at%) can be synthesized via a simple hydrothermal reaction of GO and urea [18]. N-introduction and GO reduction are achieved simultaneously under the hydrothermal reaction.
Three Dimension Functionalized Graphene
It is well known that unless the individual layers of 2D graphene are kept isolated the electrode structure, there occurs agglomeration that impairs proper functioning because of the limited availability of actual surfaces for electrochemical reaction. A highly interconnected macroporous structure together with ultralow- density are observed in 3D graphene architectures. The 3D graphene frameworks like graphene foams [19,20] is thus an efficient matrix that solves the issues associated with the 2D graphene. Three dimensional structures have many roles to play, such as maximizing the active surface area, facilitating the movement of ions through open pores and so on, while preventing the graphene sheets from restacking. The foam-like macroscopic structures of graphene/ GO are commonly produced via self-assembly method to prepare GO, which is then reduced by using strong chemical oxidants [21]. However, foam structures, produced by this method, contain defects and exhibit low electrical conductivity [22,23]. On the other hand, the Chemical Vapor Deposition (CVD) method can produce 3D graphene foams with much improved and controlled morphologies while maintaining a large surface area. In fact, free standing graphene foams can be made with high electrical conductivity and improved structural stability that is superior to the chemically derived graphene sheets [24]. Various graphene decorated models, developed from the electrochemical performance, have shown a significant improvement in capacitance due to extra and deeper redox reactions originating from the enhancement of conductivity via the defect structures and dispersion [25].
3D Graphene-CNT Functionalized with Nickel Hydroxide
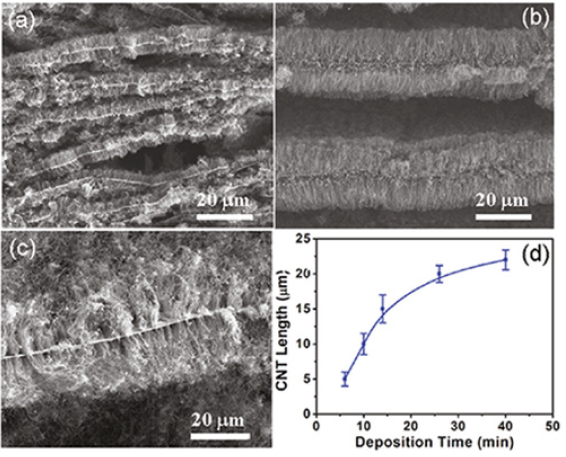
The 3D pillared Vertically Aligned Carbon Nanotubes (VACNT) graphene architectures can be made by growing vertically aligned carbon nanotubes between graphitic layers in thermally expanded Highly Ordered Pyrolytic Graphite (HOPGs) via pyrolysis of iron phthalocyanine (FePc) at 800-1000 °C under Ar/H2 [26]. Prior to CNT formation, this is treated in a tube furnace at 1000-1200 °C with a gas mixture flow of Ar and H2 bubbling through silicon tetrachloride (SiCl4) in an ambient atmosphere to produce the SiO2 coating, which is required for facilitating the growth of VACNTs [27]. The resultant VACNT/graphene 3D networks show several fold increase in mass together with few hundred times volume expansion as compared to HOPG. The FePc pyrolysis time can be varied to design required pillar length. Figure 2 shows the increase of length of the VACNT pillars with increasing the nanotube deposition time [28]. The very strong growing VACNT pillars are able to “push” the stacked graphene layers apart from each other. The CV of Ni(OH)2-coated VACNT graphene architecture (CNT length 10μm, 58wt% Ni(OH)2) in an aqueous KOH solution (2M) exhibits a pair of strong redox current peaks, arising from the Faradaic reactions of Ni(II) Ni(III). The specific capacitance of 1384Fg-1 at a scan rate of 5mVs-1 decreases to 970Fg-1 (based on the active material, i.e., Ni(OH)2) at 30mVs-1 before leveling off. The charge/discharge cycling at a relatively high current density of 21.5Ag-1 exhibits very rugged character of the material as only 4% capacity loss occurs after 20000 consecutive cycles.
Figure 2:(a-c) SEM images of the thermally expanded graphene layers intercalated with VACNTs for different pyrolysis times of 5, 10, and 30min, respectively, and (d) the VACNT pillar height as a function of the nanotube deposition time.
Functional Pillared Graphene Frameworks (FPGF)
Pillared graphene fragments in-between graphene sheets are synthesized by first applying ozone-treatment of graphene oxide [29]. Highly stable oxygen-containing functional groups are incorporated into the carbon networks through the selective ozone etching of oxygen species present on Graphene Oxide (GO). This is followed by thermal reduction process. The FPGF electrode has continuous ion transport network and high ion-accessible surface area for fast redox reaction. Thus, the system exhibits ultrahigh volumetric capacitance of 400Fcm-3. The Nyquist impedance plots shows a vertical curve of FPGF-200 (Fragment size: 200nm), which is closer to the imaginary impedance axis (Z′′). This indicates high conductivity and low internal resistance of FPGF-200. Also, the FPGF-200 is characterized by a small relaxation time constant (τ 0) of 475ms. Excellent cyclic stability is observed as 104% of its initial capacitance is retained after 10000 cycles.
Functionalized Graphene Nanosheets Decorated on CNT Networks
Functionalized graphene nanosheets/carbon nanotubes networks (G/CNTs) are produced by a simple and convenient method including chemical oxidation followed by low temperature reduction [30]. The hybrid structure comprises 1D carbon nanotubes as the continuous conductive networks and 2D functionalized graphene nanosheets as pseudocapacitive materials. The G/CNTs-200 (thermal treatment at 200 °C) electrode exhibits high specific capacitance around 200Fg−1 at a current density of 0.5Ag−1 in 6molL−1 aqueous KOH electrolyte. A symmetric device based on G/CNTs200 electrodes provides an energy density of 11.7Whkg−1. The electrochemical study exhibits retention of 102% of its initial capacitance after 20,000 cycles in 1molL−1 Na2SO4 aqueous electrolyte.
Thermally Reduced Graphene Network on Ni Foam
3D graphene foams can be prepared by hydrothermal treatment of GO and Chemical Vapor Deposition (CVD) on Ni foam [31]. Thermally Reduced Graphene Network (TRGN) deposition on Ni foam without the use of any conductive agents and polymer binders is done by using Graphene Oxide (GO) soaking and thermal reduction process. The direct and close contact between Ni foam and graphene nanosheets together in Thermally Reduced Graphene Network (TRGN) with a large amount of stable oxygen-containing groups qualifies it to maintain high specific capacitance. Repeated dipping of Ni-foam in GO suspension followed by heating in an oven at 45 °C and final heating at 300 °C under nitrogen atmosphere gives the desired product. The TRGN symmetric supercapacitor exhibits a high energy density of 30.4Whkg-1 based on the total mass of the two electrodes in 1molL-1 Na2SO4 electrolyte. The cycling stability is also excellent as it maintains the initial capacitance even after 5000 cycles.
Adenine-Functionalized Spongy Graphene
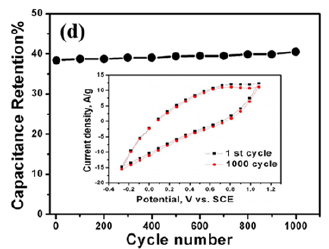
Spongy Functionalized Graphene (SFG) can be made by using freeze-drying of graphene oxide solution to prevent graphene sheets from restacking together. The procedure helps in deriving interconnected, porous 3D network crinkly sheets. Such 3D network structure aids the charge transfer kinetics by providing superior contact at the electrode/electrolyte interface. The functionalization of the graphene oxide is then performed by using adenine. The nucleophilic addition reactions between –NH2 groups of adenine and carboxylic acid or epoxy groups on the GO lead to the bonding of adenine molecules to the GO via amide linkage. Various changes of the graphene structure, such as increase of interlayer-spacing or layer scrolling, also occur. The Functionalized Spongy Graphene architecture (FSG) is obtained after annealing. The total synthesis scheme is presented in (Figure 3) [32]. Decrease in the in-plane sp2 domains and increase in the edge planes, as well as the disorder in the FG occurs due to extensive oxidation and solvothermal reduction. The specific capacitance of the FG electrode is very high (333Fg-1) at a scan rate of 1mVs-1. However, at high scan rate, the electrolyte does not get enough time to enter into the complex micropores of the electrode that result in large drop in capacitance. Excellent capacitance retention of 102% can be achieved even after 1000 cycles at 200mV/s (Figure 4).
Figure 3:Synthesis of adenine-functionalized grapheme.
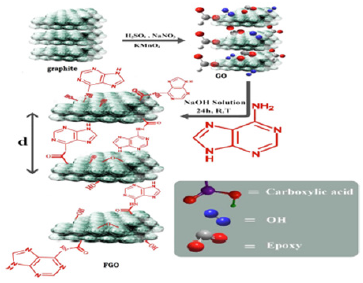
Figure 4:The first and 1000th CV cycles of the adenine-functionalized graphene electrodes. in 0.5M H2SO4 at a scan rate of 5mV/s.
Pd-Loaded Graphene Aerogel
The increase in the bulk electrical conductivity is possible by functionalization of the Graphene Aerogel (GA) with metal nanoparticles as they preferentially anchor at the otherwise electrical impeding defect sites [33]. Palladium loaded GA is fabricated in two major steps:
(1) Lyophilisation of a well-dispersed graphene oxide (GO) sheets and PdCl2/EDTA solution.
(2) Reduction of the GO aerogel with embedded Pd salt through low temperature hydrazine vapour and high temperature hydrogen separately.
Introduction of Pd nanoparticles into the 3D network structure of GA reduces the electrical resistivity from 950 to 16Ωcm. Although, Pd is a relatively expensive metal, its environmental and electrochemical stability make it a potential candidate for improving the device performance. High specific capacitance (176Fg-1 at 5mVs-1), excellent rate capability, superior columbic efficiency, and remarkable reversibility are associated with the electrodes made by using Pd doped GA [34]. Excellent cycle stability, high energy and power densities are also common to these electrodes. In the Nyquist plot, the straight line at low frequency is almost parallel to Y-axis indicating an ideal capacitive behavior of MnO2//Pd-GA asymmetric supercapacitor (in 0.1M sodium sulphate). Moreover, low internal resistivity of the electrode and good conductivity of the electrolyte are achievable. Excellent cycling stability (approx. 90% capacitance retention after 3000 cycles) makes the device very interesting.
Graphene aerogel with p-phenylenediamine and porous MnO2 composite
Functionalized graphene aerogel, synthesized via the interaction between graphene oxide and p-phenylenediamine as a functionalizing agent, is used to make composite with MnO2 for making porous electrode of supercapacitor [35]. Porous structure for functionalized graphene aerogel is decorated with flower-like microsphere of MnO2. Supercapacitor performance in 0.5M Na2SO4 confirms high energy density of 26.2Whkg−1 (at the power density of 1000Wkg−1) and excellent cycle life (94% capacitance retention after 5000 cycles).
3D porous oxygen-enriched graphene hydrogels
3D Porous Oxygen-Enriched Graphene Hydrogels (POGHs) are prepared via a one-step hydrothermal approach with graphene oxide and a tiny amount of acidic glutamic acid, which acts as carboxyl source, reductant, nitrogen dopant, as well as pore size and density regulator [36]. The as-obtained POGHs binder-free electrodes with nitrogen doping exhibit excellent electrochemical properties in 6M KOH electrolyte. Maximum capacitance of 256.5Fg−1 is observed at 0.5Ag−1, which is maintained at 91.8% even at 10Ag−1. Excellent cycling stability is evident from 100.7% capacitance retention after 10000 cycles at 10Ag−1.
3D graphitic carbon nitride functionalized graphene
Graphitic carbon nitride (g-C3N4), a two-dimensional (2D) graphite-like structure, has attractive properties, such as high nitrogen content, excellent chemical and thermal stability, special optical features, appealing electronic structure and environmentally friendly feature, which facilitate its use in energy conversion process [37]. The combination of g-C3N4 with graphene is expected to improve the conductivity and electrocatalytic performance of g-C3N4. Assembly of g-C3N4 and graphene into 3D interconnected networks is done by using one-step hydrothermal method [38]. The resulting well-defined 3D g-C3N4@G induces the hierarchical architecture, which facilitates the charge transfer and multi-way electron transfer that can effectively accelerate the electrochemical process to achieve high energy storage. 3D g-C3N4@G is prepared by hydrothermal reduction of GO sheets and g-C3N4 in their mixed aqueous dispersion and is followed by lyophilization. Teflon-lined autoclave is used at 180 °C for 12h. Composite structure is obtained by the intermolecular π–π stacking interaction between graphene layer and g-C3N4 that depends on homogenous distribution with the hydrothermal treatment. The formation of composite does not affect the electrical conductivity of graphene to appreciable extent. The surface area increases along with creation of some mesopores. The charge/discharge specific capacitance values of the g-C3N4@G based supercapacitor remains as high as 286Fg-1 at a discharge current density of 0.4Ag-1 even after 1000 cycles. The performance remains very high after 10000 cycles as well.
Optimized oxidation of graphene for designing 3-dimensional network
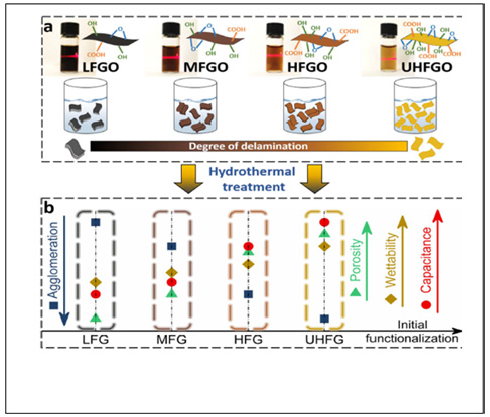
A GO precursor is mostly used to develop hydrothermally reduced GO (htrGO) structures. Surface oxygen functionalities are mainly reduced by dissociation in the form of CO2, which develops carbon vacancies on the graphene basal plane that result in the generation of defective sites and nanopores together with creation of void spaces within the network structure due to entrapment of CO2 bubbles [39]. The hydrothermally reduced GO (htrGO) structures with hierarchical pores and considerable surface area can make efficient charge storage and transport. The as-prepared GO precursors having tune able oxygen contents of 27.1, 29.5, 31.1 and 33.9 at%, respectively (named as Low-Level Oxygen- Functionalized GO (LFGO), Medium-Level Oxygen-Functionalized GO (MFGO), High-Level Oxygen-Functionalized GO (HFGO) and Ultrahigh-Level Oxygen- Functionalized GO (UHFGO) (Figure 5). The optimized three-dimensional graphene frameworks exhibit capacitance of 330Fg−1 in an aqueous electrolyte and also superior performance in various electrolytes at a device level. Furthermore, a solid-state supercapacitor with a gel electrolyte shows capacitance of 285Fg−1 with a rate capability of 79% at 20Ag−1 and capacitance retention of 93% after 20,000 cycles.
Figure 5: Schematic diagram illustrating the production of 3D htrGO frameworks with controllable functionalization. (a) Photographs of aqueous dispersions (2mgmL-1) of each GO precursor (top), The corresponding structural illustration indicates the degree of delamination increasing with the functionalization (bottom). (b) Graphical summary for the formed 3D htrGO frameworks of their relative structure and performance versus the increasing level of initial functionalization.
Three dimensional (3D) porous graphene decorated with MoO2 nanoparticles
Three Dimensional (3D) porous graphene decorated with MoO2 nanoparticles are synthesized by hydrothermal method to have random deposition of MoO2 [40]. The mass ratio of molybdenum precursor with GO was maintained at 30:1 to exhibit a higher specific capacitance and better cycling stability. The specific capacitance was around 355F/g at the current density of 0.1A/g in KOH electrolyte.
Graphene foam and zinc oxide (ZnO) electrodes-based battery like supercapacitor (Superbat)
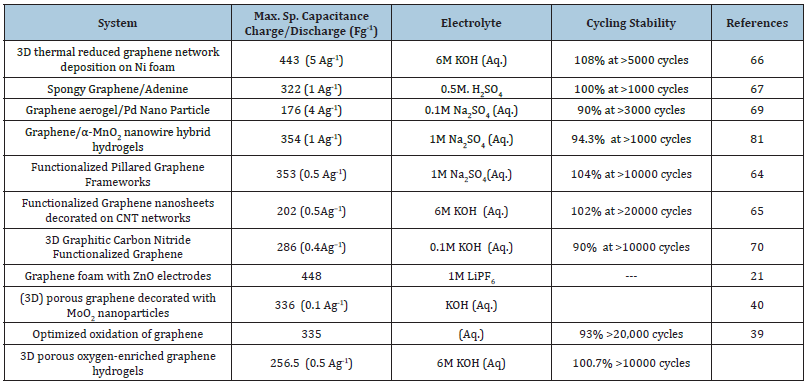
Defect structures are known to improve the electrochemical performance of superbat and prove that these electrode materials (ZnO and 3D-GF) can be used successfully for the improvement of the performance of supercapacitors. A hybrid type of energy storage device comprising graphene foam and zinc oxide electrodes exhibits both the electrochemical performance of a supercapacitor with higher power density than conventional supercapacitor, and a battery with higher energy density as compared to conventional battery. The hybrid’s improved performance can be correlated to the defective structure of the electrodes. A high specific capacitance of 448Fg-1 is obtained for the system which is due to both the quality of the synthesis and the choice of electrode and electrolyte materials [21]. Table 1 compiles the electrochemical characteristics of three-dimensional graphene structures. Three dimensional structures are expected to allow free movement of electrolyte and maintain steady capacitance value. In fact, the specific capacitance varies in the range of 176-443Fg-1. The cycling stability is mostly satisfactory graphene/graphene and graphene/CNT combination in three dimensional architectures. More than 100% of initial capacitance is retained even up to after 20000 galvanic charging/ discharging cycles.
Table 1: Electrochemical characterization of graphene in three dimensional structures.
a. Direct and close contact between Ni foam and graphene nanosheets reduce the electrode resistance and enhance the charge-transfer rate between the electrolyte and the electrode material.
b. High concentration of oxygen functional groups on the graphene provides high pseudo capacitance.
High cycle life has also become possible due to its ability to manage the strain during charge/discharge cycles. The graphene nanosheets decorated on carbon nanotubes networks has unique hybrid structure consisting of 1D carbon nanotubes and 2D functionalized graphene nanosheets. The attractive electrochemical performances exhibited are due to following reasons: Firstly, the high capacitance of Thermally Reduced Graphene Network (TRGN) on Ni-foam is attributed to the following reasons: Rapid transport of electrons has become possible due to the continuous conductive networks provided by the internal integrated carbon nanotubes that facilitates, and accordingly improves the rate performance. Secondly, the external walls of CNTs are unzipped and transformed into functionalized graphene nanosheets, which increases the specific surface area and provide substantial electrochemically active sites for electrolytic activity. Also, the surfaces of functionalized graphene nanosheets contain oxygen functionalities, which provide extra pseudo capacitance and improve the wettability of electrode materials. Choice of electrode and its preparation method also influence the capacitance values as we can see in the case of superbat.
References
- Simon P, Gogotsi Y (2008) Materials for electrochemical capacitors. Nature Mater 7(11): 845-854.
- Inagaki M, Konno H, Tanaike O (2010) Carbon materials for electrochemical capacitors. J Pow Sources 195: 7880-7903.
- Zhang LL, Zhao S, Tian XN, Zhao XS (2010) Layered graphene oxide nanostructures with sandwiched conducting polymers as supercapacitor electrodes. Langmuir 26(22): 17624-17628.
- Psarras GC (2008) Nanodielectrics: An emerging sector of polymer nanocomposites. Express Polym Lett 2(7): 460-460.
- Xia JL, Chen F, Li JH, Tao NJ (2009) Measurement of the quantum capacitance of graphene. Nat Nanotechnol 4(8): 505-509.
- Liu CG, Yu ZN, Neff D, Zhamu A, Jang BZ (2010) Graphene-based supercapacitor with an ultrahigh energy density. Nano Lett 10(12): 4863-4868.
- Wu ZS, Wang DW, Ren W, Zhao J, Zhou G, et al. (2010) Anchoring hydrous RuO2 on graphene sheets for high‐performance electrochemical capacitors. Adv Funct Mater 20(20): 3595-3602.
- Chen S, Zhu JW, Wu XD, Han QF, Wang, X (2010) Graphene oxide-MnO2 nanocomposites for supercapacitors. ACS Nano 4(5): 2822-2830.
- Mini PA, Balakrishnan A, Nair SV, Subramanian KRV (2011) Highly super capacitive electrodes made of graphene/poly(pyrrole). Chem Commun 47: 5753-5755.
- Frackowiak E, Beguin F (2001) Carbon materials for the electrochemical storage of energy in capacitors. Carbon 39(6): 937-950.
- Lin Z, Liu Y, Yao Y, Hildreth OJ, Li Z, et al. (2011) Superior capacitance of functionalized graphene. J Phys Chem C 115(14): 7120-7125.
- Sharma R, Baik J, Perera C, Strano MS (2010) Anomalously large reactivity of single graphene layers and edges toward electron transfer chemistries. Nano Lett 10(2): 398-405.
- Wang H, Maiyalagan T, Wang X (2012) Review on recent progress in nitrogen-doped graphene: Synthesis, characterization, and its potential applications. ACS Catalysis 2(5): 781-794.
- Hu Y, Sun X (2013) Chemically functionalized graphene and their applications in electrochemical energy conversion and storage. Chapt 7, Intech Open.
- Wei D, Liu Y, Wang Y, Zhang H, Huang L, et al. (2009) Synthesis of N-doped graphene by chemical vapor deposition and its electrical properties. Nano Lett 9(5): 1752-1758.
- Guo B, Liu A, Chen E, Zhu H, Fang L, et al. (2010) Controllable N-doping of graphene. Nano Lett 10(12): 4975-4980.
- Wang X, Li X, Zhang L, Yoon Y, Weber PK, et al. (2009) N-doping of graphene through electrothermal reactions with ammonia. Science 324(5928): 768-771.
- Sun L, Tian C, Tan T, Xie Y, Shi K, et al. (2012) Nitrogen-doped graphene with high nitrogen level via a one-step hydrothermal reaction of graphene oxide with urea for superior capacitive energy storage. RSC Adv 2: 4498-4506.
- Chen P, Dong X, Wang X, Wang L, Song H, et al. (2012) 3D graphene foam as a monolithic and macroporous carbon electrode for electrochemical sensing. ACS Appl Mater Interfaces 4(6): 3129-3133.
- Chen Z, Ren W, Gao L, Liu B, Pei S, et al. (2011) Three-dimensional flexible and conductive interconnected graphene networks grown by chemical vapour deposition. Nat Mater 10(6): 424-428.
- Kasap S, Kaya II, Repp S, Erdem E (2019) Superbat: battery-like supercapacitor utilized by graphene foam and zinc oxide (ZnO) electrodes induced by structural defects. Nanoscale Adv 1: 2586-2597.
- Dreyer DR, Park S, Bielawski CW, Ruoff RS (2010) The chemistry of graphene oxide. Chem Soc Rev 39(1): 228-240.
- Park S, Ruoff RS (2009) Chemical methods for the production of graphenes. Nat Nanotechnol 4(4): 217-224.
- Nardecchia S, Carriazo D, Ferrer ML, Gutierrez MC, del Monte F (2013) Three dimensional macroporous architectures and aerogels built of carbon nanotubes and/or graphene. Chem Soc Rev 42: 794-830.
- Zhu J, Childress AS, Karakaya M, Dandeliya S, Srivastava A, et al. (2016) Defect engineered graphene for high-energy- and high-power density supercapacitor devices. Adv Mater 28(33): 7185-7192.
- Huang SM, Dai L, Mau AWH (1999) Patterned growth and contact transfer of well-aligned carbon nanotube films. J Phys Chem B 103(21): 4223-4227.
- Du F, Yu DS, Dai LM, Ganguli S, Varshney V, et al. (2011) Preparation of tunable 3D pillared carbon nanotube-graphene networks for high-performance capacitance. Chem Mater 23(21): 4810-4816.
- Qu L, Zhao Y, Dai L (2006) Carbon microfibers sheathed with aligned carbon nanotubes: Towards multidimensional, multicomponent, and multifunctional nanomaterials. Small 2(8-9): 1052-1059.
- Jiang L, Sheng L, Long C, Wei T, Fan Z (2015) Functional pillared graphene frameworks for ultrahigh volumetric performance supercapacitors. Adv Energy Mater 5(15): 1500771.
- Ding B, Guo D, Wang Y, Wu X, Fan Z (2018) Functionalized graphene nanosheets decorated on carbon nanotubes networks for high performance supercapacitors. J Pow Sources 398: 113-119.
- Wu X, Yang D, Wang C, Jiang Y, Wei T, et al. (2015) Functionalized three-dimensional graphene networks for high performance supercapacitors. Carbon 92: 26-30.
- El Gendy DM, Abdel GNA, El Sherbini EEF, Allam NK (2017) Adenine-functionalized spongy graphene for green and high-performance supercapacitors. Sci Rep 7: 43104.
- Okamoto Y (2006) Density-functional calculations of icosahedral M13 (M=Pt and Au) clusters on graphene sheets and flakes. Chem Phys Lett 420(4-6): 382-386.
- Yu Z, McInnis M, Calderon J, Seal S, Zhai L, et al. (2015) Functionalized graphene aerogel composites for high-performance asymmetric supercapacitors. Nano Energy 11: 611-620.
- Gholipour RH, Ganjali MR, Norouzi P, Naderi HR (2016) Functionalized graphene aerogel with p-phenylenediamine and its composite with porous MnO2: investigating the effect of functionalizing agent on super capacitive performance. J Mater Sci: Mater Electronics 27(10): 10163-10172.
- Zhang Y, Fan S, Li S, Song Y, Wen G, (2020) 3D porous oxygen-enriched graphene hydrogels with well-balanced volumetric and gravimetric performance for symmetric supercapacitors. J Mater Sci 55: 12214-12231.
- Zheng Y, Liu J, Liang J, Jaroniec M, Qiao SZ (2012) Graphitic carbon nitride materials: controllable synthesis and applications in fuel cells and photocatalysis. Energy Environ Sci 5: 6717-6731.
- Chen Q, Zhao Y, Huang X, Chen N, Qu L (2015) Three-dimensional graphitic carbon nitride functionalized graphene-based high-performance supercapacitors. J Mater Chem A 3: 6761-6766.
- Li Z, Gadipelli S, Yang Y, He G, Guo J, et al. (2019) Exceptional supercapacitor performance from optimized oxidation of graphene-oxide. Energy Storage Materials 17: 12-21.
- Zhang L, Lin H, Zhai l, Nie M, Zhou J, et al. (2017) Enhanced supercapacitor performance based on 3D porous graphene with MoO2 J Mater Res 32(2): 292-300.
© 2021 Samui AB. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






