- Submissions

Full Text
Polymer Science: Peer Review Journal
Nanoparticles Encapsulated Cellulose Acetate Electrospun Nanofiber Membranes for Antibacterial and Microbial Filtration Applications
Landage KS1, Arbade GK2* and Bhongale CJ1
1Department of Applied Chemistry, Defence Institute of Advanced Technology, India
2Department of Metallurgical and Materials Engineering, Defence Institute of Advanced Technology, India
*Corresponding author: Gajanan K Arbade, Department of Metallurgical and Materials Engineering, Defence Institute of Advanced Technology, Girinagar, Pune- 411025, India
Submission: April 26, 2021;Published: June 15, 2021

ISSN: 2770-6613 Volume2 Issue1
Abstract
In the present study, we have prepared Titanium Oxide (TiO2) encapsulated Cellulose Acetate (CA) nanofiber membranes via electrospinning technique. Properties of the electrospun membranes were characterized by SEM, FTIR, water contact angle measurements, and antibacterial testing against common bacterial water contaminants including Escherichia coli and Bacillus subtilis. There was a little increase in the fiber diameter was observed in CA/TiO2 composite membranes as compared to that of pristine CA membranes. Also, the enhancement of hydrophilicity of CA membranes was observed upon the addition of TiO2. The photocatalytic potential of the membranes was confirmed by methylene blue dye degradation. Further, the electrospun nanofiber composite membranes were demonstrated for antibacterial filtration applications.
Keywords: Electrospinning; Antibacterial membranes; Microbial filters; Catalysis; Titanium oxide nanoparticles
Introduction
In recent years, Cellulose Acetate (CA) electrospun nanofiber membranes gained a tremendous interest in the polymer research due it broad research application. CA is a modified natural polymer produced by acetylation of cellulose, which is one of the most abundant, low cost renewable natural polymers [1]. The electrospun CA membranes have been extensively studied for its application in water treatment technologies [2,3], sensor biology [4], heavy metal separation [5], air filters [6], UV-shielding [7] biomedical and tissue engineering [8,9], and microbial purification [10]. Cellulose acetate is most commonly used polymer due to abundance, biodegradability, and good toughness with relatively low cost. TiO2 nanoparticles are well known for its antibacterial property [11,12]. In our studies we have prepared TiO2 nanoparticles by using biological approach, characterized for morphological, structural and antibacterial properties [13] and used for the fabrication of CA/TiO2 composite nanofiber membranes via electrospinning. Electrospinning is a versatile technique to fabricate the fibers of nanometer to micrometer range [14,15]. Recently, Gao et al. [16] have prepared cellulose acetate/TiO2 composites films by combination of different techniques like ultrasonic dispersion, stirring and hot-pressing etc. The prepared films contain 4wt. % to 12wt. % of TiO2 nanoparticles. These films shows antibacterial property against Escherichia coli and authors claimed these for films for its antibacterial applications. The limitation of this study is the surface area to volume ratio of the films. Attempts have been made for encapsulation of TiO2 NPs into the electrospun membranes by Das et al. [17]. In this study, researchers have demonstrated that the addition of the TiO2 NPs resulted into formation of largely interconnected porous membranes and also found that proportional enlargement in the fiber diameter upon addition of at different TiO2 concentrations ranging from 0-6.5wt. %. This study was limited to its preparation and characterizations only. Several research groups around the globe have developed different nanoparticle based composite membranes using electrospinning technology for various water purification. For instance, Kim et al. [18] have fabricated composite Polyurethane (PU) membranes containing TiO2 and Fly Ash (FA) Nanoparticles (NPs). Song et al. [19] have developed Polyethylene Glycol (PEG)-TiO2-doped Polyvinylidene Fluoride (PVDF) membranes by integration of ultrafiltration with photocatalytic property. A polyacrylonitrile-TiO2 composite adsorbent bead was prepared for the removal of heavy metal ion in aqueous solution [20].
In this context, in order to increase the surface area we have used electrospinning approach to fabricate the cellulose acetate and CA/TiO2 composite electrospun nanofiber membranes. The nanofibers in the electrospun membrane provides high surface area, porosity, and feasible modification for desired applications. In the present study, we have fabricated cellulose acetate and TiO2 nanoparticles loaded cellulose acetate electrospun nanofiber composite membranes, characterized for its properties and evaluated for its antimicrobial and photocatalytic potential. Further, these membranes were demonstrated as antibacterial filtration for water purification. The value addition of this research to the field that we have prepared the TiO2 NPs by using a biological, more ecofriendly approach [13]. We have used reproducible bacterium (Staphylococcus aureus) for the synthesis of the TiO2 NPs. These well characterized TiO2 NPs were used in this study for mentioned applications.
Materials and Methods
Materials
Cellulose Acetate (CA) of average Mn 30,000gmol-1, acetone, Dichloromethane (DCM), Dimethyl Sulfoxide (DMSO), ethanol, Dimethylformamide (DMF), acetic acid, Potassium Chloride (KCl) and Titanium Tetraisopropoxide (TIP) average Mn 284.25gmol- 1 were purchased from sigma aldrich, India. Nutrient agar and bacteriological agar powder were purchased from Himedia, India.
Bacterial cultures growth and culture maintenance
All the bacterial strains viz. Escherichia coli, Bacillus subtilis, Staphylococcus aureus, Pseudomonas aeruginosa, were obtained from National Collection of Industrial Microorganisms (NCIM), Pune, India. All the cultures were grown on nutrient agar plates and maintained on the nutrient agar slants at 4 °C. The cultures were activated by growing the in the nutrient broth medium overnight to get the cell concentration in the range of 103-104 at 37 °C prior to use in the further experiments.
Solution preparation and viscosity measurement
To prepare pristine CA solution (10wt. %) 1gm of CA was dissolved in 10ml of DMF/acetone (1:9 v/v). The composite solution CA containing TiO2 nanoparticle was prepared by incorporation of the well characterized TiO2 nanoparticles [13] to the CA solution (5wt. %) and kept for magnetic stirring for 6h to obtain a homogenous solution. The viscosities of the solutions were determined using a Brookfield digital viscometer (Model-DVprime) at room temperature. The measurements were conducted using a cone-and-plate geometry with a diameter of 25mm and a cone angle of 5.4∘ at room temperature. Steady state shear viscosity was measured at shear stresses in the range 0.24-370Pa.
Membrane fabrication by electrospinning
Measured 10ml of CA solution was taken into each metallic needle syringe 24G, syringes were fixed horizontally on the syringe pump, attach with high voltage supply (Nanomate, India). Electrospining was performed at room temperature with an applied voltage of 10kV, flow rate 3ml/h and tip to collector distance was 10cm. Electrospun fibers were deposited on a grounded stationary metallic plate collector covered by aluminum foil. The membranes were dried in vacuum desiccator and stored for further use.
Membrane Characterization
Scanning electron microscopy
Field emission scanning electron microscopy (FE-SEM ZESIS, Germany) was used to investigate the membrane morphology and diameter of the fibers in the fabricated membranes. Samples were cut from the bare membranes and coated gold/palladium before imaging. The average fiber diameter was determined by taking maximum measurements in the SEM images, using the ImageJ software (National Institute of Health, Bethesda, MD, U.S.A.). Average fiber diameters were mentioned with standard deviations.
Surface wettability of the membranes by water contact angle measurement
To study the surface wettability of the membranes, the water contact angle of CA and CA/TiO2 nanofiber membranes was measured by sessile drop method as described in our previous studies; Arbade et al. [21]. The water contact angle was measured at room temperature on a contact angle goniometer (KRUSS, Germany) equipped with a digital camera. A drop (10μl) of deionized water was dropped onto the each dried electrospun membrane with the help of attached micro syringe, in an atmosphere of water air. Minimum 3 measurements were taken and averaged to get a reliable value of contact angle.
Fourier transform infrared spectroscopy (FTIR)
The chemical interaction of the TiO2 with cellulose acetate in the nanofiber was studies by FTIR spectroscopy. The spectra were recorded in the wave number between 500 and 4500cm−1 at a resolution of 4cm−1 with an average of 25 scans.
Antimicrobial testing of CA and CA/TiO2 membranes
The antimicrobial activities of the electrospun nanofiber membranes was tested against the different bacterial strains involved in water contamination viz. Escherichia coli and Bacillus subtilis by disk diffusion method as described in our previous studies [22]. Simply, electrospun membranes were cut into 1.0×0.5cm pieces and sterilized by UV sterilization for 20min. The sterile nanofiber membranes were the aseptically kept on the agar plates seeded with specific bacterial cultures. The plates were then incubated in a bacteriological incubator at 37 °C for 24h, after incubation period plates were observed for the Zone of Inhibition (ZOI) around the membrane. The zone of inhibitions were measured and recorded. The experiment were repeated thrice and average ZOI with standard deviation was presented.
Design of microbial filtration assembly and evaluation of microbial filtration
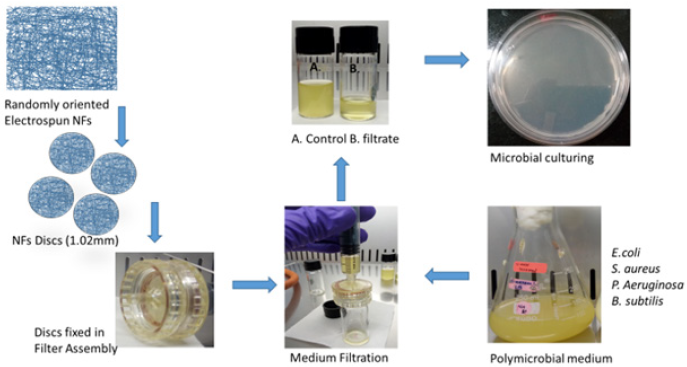
In order to design a microbial filtration assembly the electrospun membranes were cut into circular discs with 1cm diameter and fixed into the syringe filters (Tarson, India). The thickness of the membrane was ~1.0mm, the membrane thickness in the assembly was increased by putting discs over one another. The complete assembly and electrospun membrane discs were sterilized by autoclaving and UV sterilization respectively before fixing the membrane disks into the filtration assembly. The filtration ability of the electrospun membranes were evaluated by filtering the nutrient broth medium containing actively growing different bacterial cultures. The (Figure 1) represents the typical experimental design and evaluation of the membranes as microbial filters. The filtrate samples were collected in separate sterile glass vials aseptically in biosafety cabinet (Euroclone, Germany). The filtrate samples were the plated on the nutrient agar plates and plates were incubated at 37 °C in a bacteriological incubator overnight to study the growth of the bacterial count in the filtrate. The number of colonies on the agar plates against control were measured to quantify the efficiency of the filtration property of the CA and TiO2 loaded electrospun nanofiber membranes. The experiments were repeated thrice, and the average number of bacterial colonies were mentioned with standard deviations.
Figure 1: Typical experimental design and evaluation of the membranes as microbial filters. Thickness of the membrane ~1.02mm and disc diameter 1.0cm.
Photocatalytic activity of the composite membranes
In order to measure the photocatalytic activity, a 300W mercury lamp was used as the simulated UV source. Photocatalytic capacity of electrospun nanofiber membrane was evaluated using aqueous Methylene Blue (MB) solution. Composite membranes containing TiO2 nanoparticles were placed into a MB aqueous solution (10mL). Before light irradiation, the samples were immersed with MB aqueous solution in quartz reactor at room temperature for 1h to establish adsorption equilibrium. Samples were taken at regular intervals of time (60min), and the concentration of the dye was measured by recording its absorbance at 500nm-800nm with a UV–visible spectrophotometer (Biospectrometer, Eppendorf, Germany).
Results and Discussion
Viscosity measurement
The viscosity of the pristine CA solution was observed ~2205cP and for CA/TiO2 composite membranes it was observed ~1411cP, despite of the difference in the viscosities it has not affected the fiber formation during the electrospinning.
Membrane appearance, morphology, and average fiber diameter in the membranes
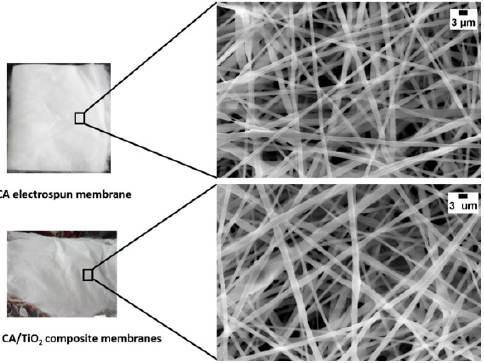
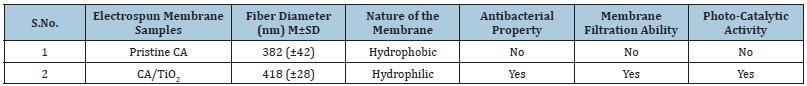
The (Figure 2) shows the appearance of the membranes and SEM mages of the nanofiber membrane. Smooth, uniform, randomly oriented and bead free nanofibers were observed for CA as well as CA/TiO2 composite membranes. The average fiber diameter of the nanofibers in the membrane of CA was observed ~382nm (±42)nm and for CA/TiO2 composite membranes it was found ~418 (±28) nm. Here, increase in the average fiber diameter was observed upon addition of the TiO2 nanoparticles to the pristine CA membranes.
Figure 2:SEM images of CA/TiO2 composite electrospun nanofiber membranes. The left side of figure shows the digital images of the electrospun membranes (Thickness ~1.0mm) and right side of the figure shows the SEM images of the respective nanofiber membranes. (Scale bar 3μm).
Fourier transform infrared spectroscopy (FTIR)
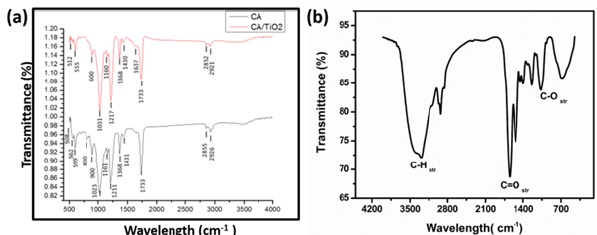
(Figure 3a) presents the FTIR spectra of pristine and TiO2 loaded electrospun nanofiber membranes and (Figure 3b) presents a spectra of TiO2 nanoparticles. It can be seen that all the samples showed the characteristic peaks of CA at 2926cm-1 for -C-H, 1733cm- 1 for >C-O and 1211cm-1 and 1023cm-1 for-C-O- respectively. Similar results were obtained elsewhere [16,23]. After the incorporation of TiO2 NPs, the intensity of peaks for –OH at -C-H at 2923cm- 1 remained same, while those for >C-O at 1736cm-1 and -C-O- at 1211cm-1 and 1021cm-1 obviously were stronger as the content of TiO2 increased, which revealed that the introduction of TiO2 did not affect the hydrophilic property of CA membranes.
Figure 3:FTIR spectra of (a) CA and TiO2 loaded electrospun nanofiber membranes and (b) TiO2 nanoparticles.
Surface wettability of the membranes
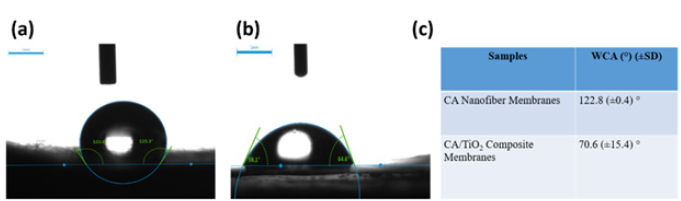
Figure 4a & 4b presents representative digital images of water contact angles of CA electrospun nanofiber membranes and CA/ TiO2 loaded composite CA electrospun nanofiber membranes. The static water contact angle of CA nanofiber membrane was observed 122.8 (±0.4)° and that for CA/TiO2 composite membranes was 70.6 (±15.4)°. The pristine CA membranes were hydrophobic in nature however upon addition of TiO2 nanoparticles, composite membranes showed hydrophobic nature. The similar properties of the electrospun membranes were reported by Gao Ying et al. [16].
Figure 4: Water contact angle of (a) CA electrospun nanofiber membranes and (b) CA/TiO2 composite electrospun nanofiber membranes and (c) table of results for WCA of the various electrospun membranes.
Antimicrobial testing
In vitro antibacterial testing was performed by disc diffusion method. The common bacterial water contaminant strains were used in the present study. Figure 5 shows the representative digital images of agar plates, where CA and TiO2 loaded electrospun nanofiber membranes were placed in presence of bacterial strains. The bacterial growth inhibition was observed as a clear circle around the membrane sample. The pristine CA membranes do not show any antibacterial inhibition activity for both bacterial strains used, whereas the TiO2 loaded CA membranes showed clear Zones of Inhibition (ZOI) around it in against both the bacterial strains. Table 1 presents the quantitative measurements of the inhibition zones. Higher ZOI value was observed against Escherichia coli as compared to that of Bacillus subtilis.
Figure 5:Representative digital images of antibacterial testing of CA and TiO2 loaded CA electrospun nanofiber membranes. The clear circles around the membrane on the agar plates shows the inhibitory activity of the TiO2 loaded membranes against common water contaminants like Escherichia coli and Bacillus subtilis.
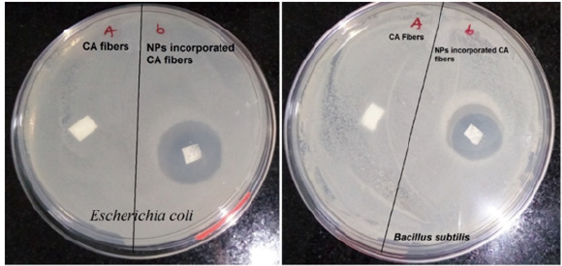
Table 1: Bacterial zone of inhibition observed around the membranes on nutrient agar plates against common bacterial water contaminants.
Evaluation of membranes for microbial filtration
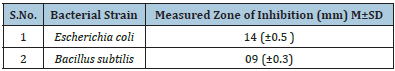
The contaminated water contains not only chemical pollutants but also contains biological contaminants. Biological contaminants: Bacteria, Fungi, protozoa, virus etc. The chemical contaminants include heavy metals (Pb2+, Cu2+, Hg2+and Cd2+) etc. In the present study, filtrate samples were used to analyze the growth of the bacteria and evaluation of ability of the membranes to purify the water samples for biological contaminants. In the Figure 6, it was clearly observed that, the sample before filtration shows complete mat bacterial growth on the agar plate. At highest membrane thickness, there was no microbial growth was observed in filtrate. But, when the swab from the membrane used for filtration was observed microbial growth. It shows that the microbes were adsorbed on the surface of the electrospun nanofiber membranes (as not able to pass the membrane) and hence no microbial growth was observed in filtrate samples but observed on the filter samples. The physical entrapment of the bacteria is the mechanism behind this observation. The samples were also filtered through 0.35 micron filters and considered as control and found similar results.
Figure 6:Digital images of the nutrient agar plates with different samples after incubation of 24h at 37 °C. A: polymicrobial culture B-D: Filtrates with different membrane thickness E: Membrane swab for polymicrobial culture and F: Membrane swab for Escherichia coli.
Photocatalytic activity of the TiO2 loaded CA electrospun membranes
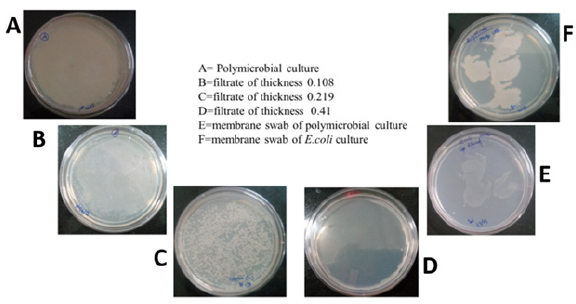
Photocatalytic activity of the CA/TiO2 composite nanofiber membrane was compared by the photocatalytic degradation of methylene blue under the irradiation of UV light. As shown in Figure 7 the intensity of the absorption for all samples decreased with increase in the incubation showing the extent of MB dye degradation. But the intensity of the absorption for all samples at 665nm was the most intensive at different degradation time, which showed methylene blue had a characteristic absorption peak at 665nm and the intensity of the absorbance at 665nm was proportional to the concentration of methylene blue in the solution (Table 2). It was observed that the degradation of MB have very small difference at time 60min and 90min. The reason behind this is that as the dye concentration increases, more and more dye molecules are adsorbed on the surface of the TiO2 NPs. The presence of large amount of dye results into the lack of direct contact of nanoparticles with the holes or the hydroxyl radicles [24]. The other reasons may include formation of the by-products during the degradation of mother dye molecules and UV screening of the MB [24,25].
Figure 7: UV-visible spectra of photocatalytic activity shown by CA/TiO2 composite electrospun membranes for methylene blue.
Table 2: Properties of the electrospun nanofiber membranes
Conclusion
Cellulose acetate and cellulose acetate composite nanofiber membranes were fabricated by electrospinning. All the nanofibers were bead free, uniform and with smooth surface morphology. Upon loading of the TiO2 nanoparticles the surfaces of the composite membranes were hydrophilic in contrast to CA membranes which are hydrophobic in nature. The antibacterial potential of the fabricated samples, composite membranes show good antibacterial activity against common bacterial water contaminants. This shows the potentiality of these membranes for filtration applications. The water filtration evaluation experiments shows the removal of bacterial species from the polymicrobial broth samples, with the maximum thickness of ~3mm membrane disks. Further, these electrospun nanofiber membranes can be used as membranes in the water purification systems. Further, we are extending these studies for the separation of heavy metal impurities from the water.
Acknowledgement
GA would like to thank Defence Institute of Advanced Technology, Pune, India for providing research fellowship. Authors are thankful to Dr. T. U. Patro for extending the required facility to conduct these study at polymer laboratory. We also thank to the reviewers for their critical comments and helpful suggestions.
References
- Mikaeili F, Gouma PI (2018) Super water-repellent cellulose acetate mats. Sci Rep 8(1): 1-8.
- Goetz LA, Naseri N, Nair SS, Karim Z, Mathew AP (2018) All cellulose electrospun water purification membranes nanotextured using cellulose nanocrystals. Cellulose 25(5): 3011-3023.
- Sato A, Wang R, Ma H, Hsiao BS, Chu B (2011) Novel nanofibrous scaffolds for water filtration with bacteria and virus removal capability. J Electron Microsc (Tokyo) 60(3): 201-209.
- Baptista A, Ferreira I, Borges J (2013) Cellulose-based bioelectronic devices. Cellulose-Medical, Pharmaceutical and Electronic Applications pp. 67-82.
- Tian Y, Wu M, Liu R, Li Y, Wang D, et al. (2011) Electrospun membrane of cellulose acetate for heavy metal ion adsorption in water treatment. Carbohydr Polym 83(2): 743-748.
- Nicosia A, Keppler T, Muller FA, Vazquez B (2016) Cellulose acetate nanofiber electrospun on nylon substrate as novel composite matrix for efficient, heat-resistant, air filters. Chem Eng Sci 153(1): 284-294.
- De Mendoza JGH, Gutierrez J, Tercjak A (2020) Transparent and flexible cellulose triacetate-TiO2 nanoparticles with conductive and UV-shielding properties. J Phys Chem C 124(7): 4242-4251.
- Rodríguez K, Gatenholm P, Renneckar S (2012) Electrospinning cellulosic nanofibers for biomedical applications: Structure and in vitro Cellulose 19(5): 1583-1598.
- Majumder S, Sharif A, Hoque ME (2020) Electrospun cellulose acetate nanofiber: Characterization and applications. Advanced Processing, Properties, and Applications of Starch and Other Bio-Based Polymers pp. 139-155.
- Carpenter AW, Francois C, Wiesner MR (2015) Cellulose nanomaterials in water treatment technologies. Environ Sci Technol 49(9): 5277-5287.
- Evans P, Sheel DW (2007) Photoactive and antibacterial TiO2 thin films on stainless steel. Surf Coatings Technol 201(22-23): 9319-9324.
- Lin H, Xu Z, Wang X, Long J, Su W, et al. (2008) Photocatalytic and antibacterial properties of medical-grade PVC material coated with TiO2 J Biomed Mater Res Part B Appl Biomater 87(2): 425-431.
- Landage KS, Arbade GK, Khanna P (2020) Biological approach to synthesize TiO2 nanoparticles using Staphylococcus aureus for antibacterial and anti-biofilm applications. J Microbiol Exp Res 8(1): 36-43.
- Arbade GK, Srivastava J, Tripathi V, Lenka N, Patro TU (2020) Enhancement of hydrophilicity, biocompatibility and biodegradability of poly (ε-caprolactone) electrospun nanofiber scaffolds using poly (ethylene glycol) and poly (L-lactide-co-ε-caprolactone-co-glycolide) as additives for soft tissue engineering. J Biomater Sci Polym Ed 31(13): 1648-1670.
- Arbade GK, Umasakar T (2019) Biocompatible polymer based nanofibers for tissue engineering. Advances in Sustainable Polymers pp. 43-66.
- Gao Y, Wang X, Li X, Dai H (2020) An antibacterial composite film based on cellulose acetate/TiO2 New J Chem 44(47): 20751-20758.
- Das C, Gebru KA (2017) Cellulose acetate modified titanium dioxide (TiO2) nanoparticles electrospun composite membranes: Fabrication and characterization. J Inst Eng Ser E 98(2): 91-101.
- Kim HJ, Pant HR, Kim JH, Choi NJ, Kim CS (2014) Fabrication of multifunctional TiO2-fly ash/polyurethane nanocomposite membrane via electrospinning. Ceram Int 40(2): 3023-3029.
- Song H, Shao J, He Y, Liu B, Zhong X (2012) Natural organic matter removal and flux decline with PEG-TiO2-doped PVDF membranes by integration of ultrafiltration with photocatalysis. J Memb Sci 405-406: 48-56.
- Kim HT, Lee CH, Shul YG, Moon JK, Lee EH (2003) Evaluation of PAN-TiO2 composite adsorbent for removal of Pb(II) ion in aqueous solution. Sep Sci Technol 38(3): 695-713.
- Arbade GK, Kumar V, Tripathi V, Menon A, Bose S, et al. (2019) Emblica officinalis-loaded poly (ϵ-caprolactone) electrospun nanofiber scaffold as potential antibacterial and anticancer deployable patch. New J Chem 43(19): 7427-7440.
- Arbade GK, Jathar S, Tripathi V, Patro TU (2018) Antibacterial, sustained drug release and biocompatibility studies of electrospun poly (ε-caprolactone)/chloramphenicol blend nanofiber scaffolds. Biomed Phys Eng Express 4(4): 045011.
- Serbanescu OS, Pandele AM, Miculescu F, Voicu SI (2020) Synthesis and characterization of cellulose acetate membranes with self-indicating properties by changing the membrane surface color for separation of Gd(III). Coatings 10(5).
- Tayeb AM, Hussein DS (2015) Synthesis of TiO2 nanoparticles and their photocatalytic activity for methylene blue. Am J Nanomater 3(2): 57-63.
- Che Ramli ZA, Asim N, Isahak WNRW, Emdadi Z, Ludin NA, et al. (2014) Photocatalytic degradation of methylene blue under UV light irradiation on prepared carbonaceous TiO2. Sci World J pp. 1-8.
© 2021 Arbade GK. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






