- Submissions

Full Text
Polymer Science: Peer Review Journal
Adsorption with Nanoclay: Kinetics and Isotherm Studies
Kalotra S1, Sangal VK2 and Mehta R1*
1Department of Chemical Engineering, Thapar Institute of Engineering & Technology, India
2Department of Chemical Engineering, Malaviya National Institute of Technology, India
*Corresponding author:Rajeev Mehta, Virginia Tech-TIET Center of Excellence for Emerging Materials, Thapar Institute of Engineering & Technology, Patiala 147004, India
Submission: February 12, 2021;Published: March 05, 2021

ISSN: 2770-6613 Volume1 Issue4
Abstract
In the present study, Cloisite® 15A nanoclay has been used to remove Reactive Black 5 dye (RB5) from an aqueous solution. Cloisite® 15A was nano-dispersed in double distilled water. The degree of dispersion was determined by two techniques i.e Transmission electron microscopy and X-ray diffraction. Different parameters like adsorbent dose, time, pH, initial dye concentration, and temperature have been studied to identify the efficiency of Cloisite® 15A adsorbent to remove RB5 by batch mode. The removal percentage decreased with an increase in RB5 concentration. The optimum values for RB5 were found as 0.35g of adsorbent dose, pH 5, and 35min of contact time. The isotherm study was conducted by Langmuir, Freundlich, and Tempkin models. The equilibrium data fitted well to Freundlich isotherm model. The kinetic study was done with pseudo-first and second-order models and it was determined that kinetic followed pseudo-second order with the highest correlation coefficients (0.99997 at 30 °C, 0.99997 at 35 °C, and 0.99999 at 40 °C). The adsorption results show that the Cloisite® 15A adsorbent had an overall efficiency of >99% for RB5 removal in less than 35 minutes.
Keywords: Adsorption; Nanoclay; Reactive black 5; Kinetics; Isotherms
Abbreviations: RB5: Reactive Black 5; MtMIO: Montmorillonite Clay Modified with Iron Oxide; TEM: Transmission Electron Microscopy; XRD: X-Ray Diffraction; MPSD: Marquardt’s Percent Standard Deviation
Introduction
Rivers and lakes water contamination with dyes causes serious damage to wild and human
life. Currently there are more than ten thousand dyes commercially available [1,2] and the
yearly production of dyes is more than 7x105 tones across the world [3]. From manufacturing
operations, around 2% of the dyes produced are discharged in effluent [4]. In various
industries such as paper printing, textile, pharmaceutical, electro plating, leather, rubber and
cosmetics dyes are extensively used [5]. There are different types of dyes available: cationic
include basic dyes, anionic include acid, direct and reactive dyes, and non-ionic include
dispersive dyes [2,6]. The most common type of dyes are reactive dyes [7-10]. Textile industry
is the topmost consumer of reactive dyes and releases toxic effluent containing there dyes. Dye
contamination causes serious environmental problems and is carcinogenic to human being
[11]. It is very important to completely remove dye from textile effluents before discarded
into wastewater [12]. Adsorption is the most common choice to remove dyes because of its
ability to remove dye contaminations, ease of operation, inexpensive and simple in design [4].
Adsorption is a surface phenomenon and is suitable for both continuous and batch processes
[13]. Nanoclay has been used for wastewater treatment as an adsorbent due to its nanometer
scale size, large specific surface area, layered structure, chemical and mechanical stability,
high cation exchange capacity and a variety of surface and structural properties [10,14,15].
Organically modified clay has a great potential for the removal of textile dye through adsorption
[10]. The behavior of adsorption of the RB5 onto organoclay (Cloisite® 10A and Cloisite® 15A)
has been studied by Yang et al. [16]. They found that nanoclay has high removal efficiency
for the treatment of textile dyes such as Direct Red 80, C.I. Acid Red 266, Reactive Blue 19,
Disperse Red 62 and Basic Red 2. Chen et al. [17] reported the adsorption capacity of soil
nanoclays for Crystal Violet and Methylene Blue dyes. They observed that the soil nanoclays adsorb both Crystal Violet and Methylene Blue dyes efficiently. The
ability of organoclays (hexadecyltrimethylammonium-bentonite)
has been investigated to remove Reactive Red 141 dye by Elemen
et al. [10]. It was found that 80% of the dye color was removed by
using organoclay. The study suggested that organoclay is a suitable
adsorbent for the removal of reactive dye from wastewater. Shen
et al. [18] reported that nanoclay (polydiamethylammoniumbentonite)
can be used to eliminate reactive dyes effectively. Cottet
et al. [19] determined that the adsorption of MB (Methylene Blue)
onto Montmorillonite Clay Modified with Iron Oxide (MtMIO)
was complete within 4h and the maximum adsorption capacity
of MtMIO was 71.12mg/g. The adsorption of reactive navy blue
SP-BR dyes onto organo-montmorillonite was studied by Rasouli
et al. [20]. Adsorption of Reactive Blue 29 dye using modified
nanoclay has also been investigated by Jamshidi et al. [21] and the
results revealed that 84% dye removal was observed with 6g/L of
adsorbent, in only 2min.
All these studies involved the use of nanoclays as adsorbents for
various dyes. However, the clay particles used were of micron size.
In the present study, use of Cloisite® 15A as an adsorbent for removal
of RB5 has been accomplished by dispersing the clay at nano scale.
It is hypothesized that if nanoclay is in an exfoliated state or even
in intercalated state with only a few layers of clay platelets, it will
have very high surface area and will act as an efficient adsorbent
at very low concentration of the adsorbent. In the present study,
adsorption of Reactive Black 5 dye onto dispersed Cloisite® 15A has
been investigated. The various operational parameters investigated
are contact time, pH, temperature, amount of adsorbent and initial
dye concentration. Further, the kinetics and thermodynamic aspects
of the adsorption process has been studied.
Experimental
Materials
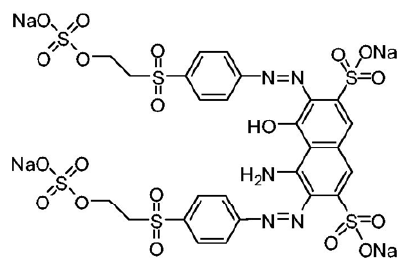
Cloisite® 15A nanoclay was purchased from Southern Clay Products (United States). As per supplier information, Cloisite® 15A had been modified with dimethyl dihydrogenated tallow quaternary ammonium modifier and the concentration of modifier is 125meq/100g of clay. The structure of the modifier is presented in (Figure 1) [22]. Reactive Black 5 dye was purchased from Sigma Aldrich (India). In (Figure 2), the structure of RB5 is shown [23]. The molar mass of RB5 is 991.83g/mol and the molecular formula for RB5 is C26H21N5Na4O19S6. The maximum adsorption wavelength (λmax) of RB5 was determined to be 597nm.
Preparation of adsorbent
Cloisite® 15A is an organically modified montmorillonite nanoclay with a quaternary ammonium salt (Figure 1). Absorbent was prepared by dispersing Cloisite® 15A in 50mL of double distilled water by using ultrasonic probe for 10min. Then, this mixture was homogenized using a homogenizer for 4min at 10,000rpm. Dispersion of clay at nano scale provides highest removal efficiency which cannot be attained from neat clay. The reason is their ultrafine particle size and large surface area.
Figure 1: Cloisite® 15A nanoclay modifier [22].
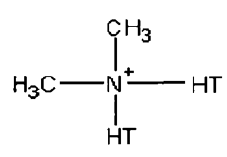
Figure 2: Structure of reactive black 5 dye [23].
Experimental procedure
A stock solution of 1g/L of RB5 dye was prepared in double distilled water. This solution was further diluted as per the experimental plan. The parameters studied were the adsorbent dosage, temperature (20 to 40 °C), contact time (upto 35min) and initial dye concentration (50 to 300mg/L). Experiments were conducted in a 250mL stoppered conical flasks containing 100mL of dye solution of known pH and adsorbent. The samples were placed in a shaking incubator at a constant speed of 150rpm and at a constant temperature for a maximum of 35min (after 35min the removal was almost constant). After that the adsorbate and the adsorbent were separated by filtration and the absorbance was analyzed using a UV/VIS spectrophotometer. The remaining dye concentration was determined by using the calibration curve. The percentage of RB5 removal was calculated using the following equation:
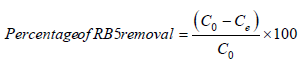 (1)
(1)
where, C0 (mg/L) is initial RB5 concentration and Ce (mg/L) is the equilibrium RB5 concentration.
Results and Discussion
Characterization of cloisite® 15A nanoclay
The structure and morphology of Cloisite® 15A were investigated by using X-Ray Diffraction (XRD) and that of the adsorbent solution by Transmission Electron Microscopy (TEM) technique. The XRD pattern of Cloisite® 15A nanoclay is shown in (Figure 3). The XRD was performed on a diffractometer system XPERT-PRO in the 2Ɵ range of 2° to 50°. Cloisite® 15A have three peaks at 2Ɵ=3.6°, 21.9° and 36.07°. The d001 interlayer spacing for Cloisite® 15A corresponding to 2Ɵ=3.6° is 2.44nm. TEM of a sample prepared using 0.35g of dispersed Cloisite® 15A is shown in (Figure 4). The figure shows that the nanoclay intercalation is present and presence of tactoid structures can be seen. The interlayer spacing of dispersed Cloisite® 15A is about 4nm. This value is significantly greater than that of pristine Cloisite® 15A (2.44nm). This suggests that during the adsorption process, the adsorbent is able to intercalate in between the clay layers, and thereby push the clay layers apart to a greater extent.
Figure 3: XRD pattern of Cloisite® 15A nanoclay.

Figure 4: TEM image of Cloisite® 15A nanoclay after adsorption.

Effect of adsorbent
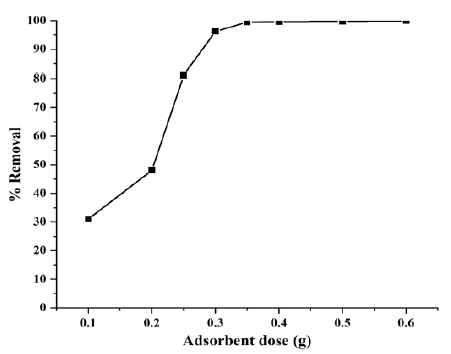
The adsorbent effect on RB5 by Cloisite® 15A nanoclay was studied for starting concentration C0=50mg/L, T=30 °C, pH=5.6 and time=50min. (Figure 5) shows the effects of adsorbent dosage on the removal of RB5. It was found that the removal of RB5 by Cloisite® 15A nanoclay increases with an increase in the adsorbent dose (m) up to 0.35g. On further increases in m, there is very little change in RB5 removal %, peaking at a removal efficiency of 99.7% for m=0.35g. Adsorption of RB5 is enhanced with an increase in the adsorbent dosage up to these levels, because of availability of larger surface area and adsorption sites. At lower dosages of the adsorbents, the adsorbent surface becomes saturated with RB5 and a larger residual RB5 concentration remains in the solution. At a dosage of 0.35g of Cloisite® 15A nanoclay for C0=50mg/L, in time duration of 50min equilibrium conditions exist for the adsorbate-adsorbent system with almost no increase in the RB5 uptake by Cloisite® 15A nanoclay.
Figure 5: Effect of adsorbent dosage on the color removal by Cloisite® 15A at T=30 °C, initial pH=5.6, C0=50mg/L and t=50min.
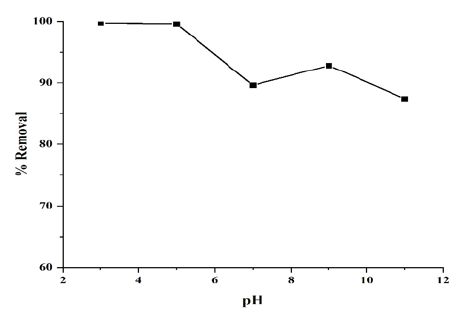
Effect of initial pH
pH is a one of the most important factor for the adsorption of dye. The pH study was varied from 3 to 11. The pH of the RB5 solution was adjusted by adding 1 N aqueous solution of either HCl or NaOH. The maximum removal of 99.7% and 98.3% were attained at pH=3 and pH=5, respectively with C0=50mg/L and T=30 °C. At a high pH, the percentage of RB5 removal was lower while at low pH solution the percentage of RB5 removal was higher because at higher pH dye precipitated, but at lower pH the active sites were free. At higher pH, the adsorbent surface negatively charged by adsorbed hydroxyl ions whereas at lower pH adsorbent surface positively charged by adsorbed hydrogen ions. The pH effect on RB5 adsorption by Cloisite® 15A is shown in (Figure 6).
Figure 6:Effect of initial pH on color removal by Cloisite® 15A.
Effect of initial RB5 dye concentration
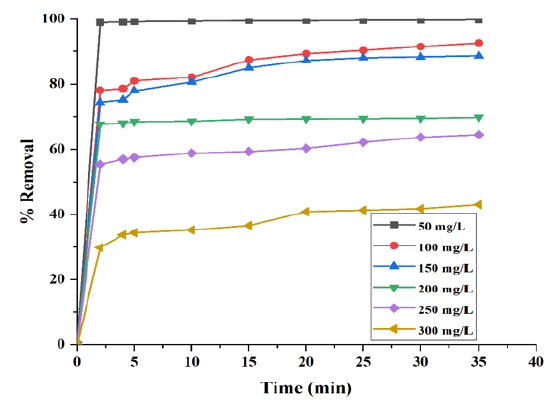
The effect of initial RB5 dye concentration (50mg/L to 300mg/L) on the removal of RB5 dye is shown in (Figure 7). The results show that % adsorption of RB5 decreased when the initial RB5 dye concentration was increased. This may be attributed due to the adsorbent surface saturation.
Figure 7: Effect of initial dye concentration on the color removal by Cloisite® 15A (T=35 °C, C0=50 to 300mg/L, adsorbent dose (m)=0.35g, t=35min, pH=5).
Kinetics of adsorption
There are many kinetic models which are used to describe the adsorption processes. Pseudo-first order and pseudo-second-order kinetic models are the most commonly used ones. Kinetic constants have been calculated by taking different C0 values (50-250mg/L), optimum adsorbent dosage, optimum pH, temperature (30°, 35° and 40 °C) and contact time (0-35min). The amount of dye adsorbed, qe (mg/g) is determined by the equation:
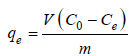 (2)
(2)
where C0 (mg/L)=initial concentration dye; Ce (mg/L)=equilibrium concentration; V (liter)=solution volume; m=optimum adsorbent dosage. The equation for pseudo-first-order model is given by:
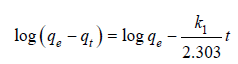 (3)
(3)
where, qe (mg/g)=amount of RB5 adsorbed at equilibrium; qt (mg/g)=RB5 adsorbed at time t ; k1 (1/min)=rate constant [2]. The pseudo-second-order equation is presented by:
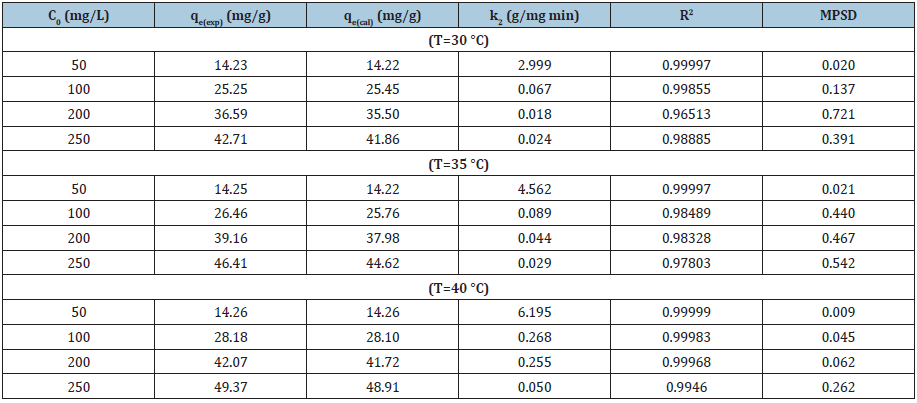
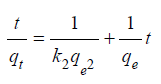 (4)
(4)
where k2 (1/min)=rate constant (pseudo-second-order). The theoretical value of adsorbed dye, qe (mg/g) can be calculated from pseudo-second-order equation [10].
Marquardt’s Percent Standard Deviation (MPSD) is an error function and has been used to find the most appropriate kinetic model [24]. The pseudo-second-order model fitted well with all experimental data and achieved highest correlation coefficients as shown in (Table 1). k2 and qe(cal) are determined from the intercept and the slope from a linear plot of t/qt vs t. The pseudo-second-order model fitting with the experimental data is shown in (Figure 8). The highest correlation coefficients R2 (R2=0.99997, 0.99997 and 0.99999) values obtained at temperature 30 °C, 35 °C and 40 °C (C0=50mg/L). MPSD error values are very small for pseudo second order model. The results of second order model shows that at T=35 °C and 40 °C, the qe values increases with an increase in the C0 whereas k2 values decreases with an increase in C0 but k2 value again increases with C0=250mg/L at T=30 oC. After the kinetic model and its parameters have been ascertained, the equilibrium model and its parameters have been studied in the following section.
Figure 8: Kinetic study of RB5 onto Cloisite® 15A nanoclay. T=(a) 30°, (b) 35° and (c) 40 °C, adsorbent amount (0.35g).
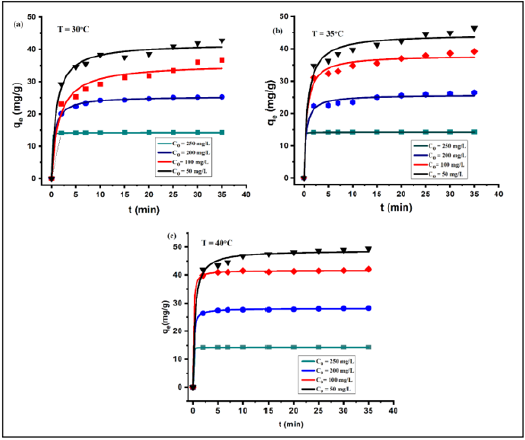
Table 1:Pseudo second order model by nonlinear method.
Isotherm study
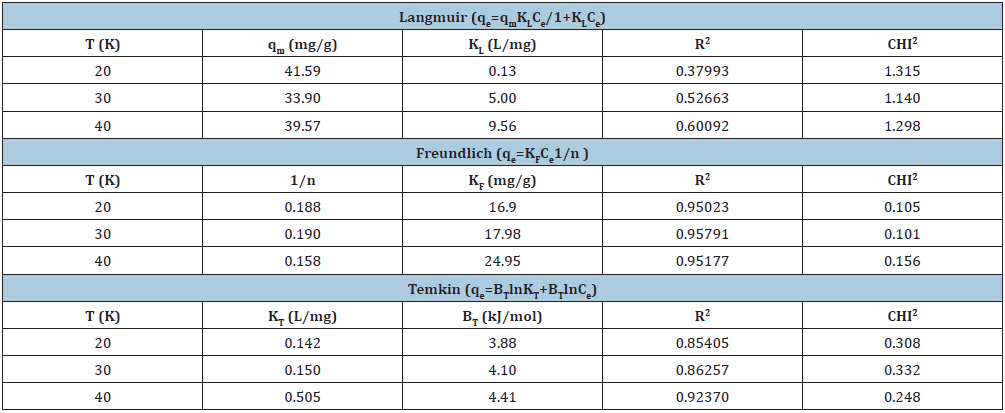
Langmuir, Freundlich and Temkin are the three commonly used isotherm models and they were studied to describe the RB5 adsorption at three different temperatures: 20 °C, 30 °C and 40 °C, at optimum pH, optimum amount of adsorbent with different C0 values in the range of 50 to 300mg/L. After 30min the adsorbent separated from the RB5 dye solution. The uptake, qe (mg/g), was calculated. (Table 2) shows the isotherm parameters of various models like Langmuir, Freundlich and Temkin isotherm model. R2 (correlation coefficient) and CHI2 (error function) are the two parameters of these three models. CHI2 is the error function to find out the best fit model. From (Table 2), it is clear that there are very small differences in CHI2 values for Freundlich isotherm model at temperature 20 °C, 30 °C and 40 °C. The equilibrium adsorption of RB5 was studied to calculate the adsorption capacity (qm), Langmuir constant (KL), Freundlich constant (KF), heterogeneity factor (1/n) and Temkin constants (KT and BT). Temperature has a major effect on the adsorption capacity of the adsorbent. The temperature effect on RB5 adsorption has been studied at 20 °C, 30 °C and 40 °C. The KF value increases with an increase in temperature and it shows that adsorption is endothermic in nature. To determine the suitability of the isotherms, the R2 values at different temperatures were analyzed. The adsorption isotherms were well fitted by the Freundlich isotherm model. The data in the (Table 2) show that Freundlich model obtain highest R2 values (R2=0.95023, 0.95791, 0.95177) and lowest CHI2 values (0.105, 0.101, 0.156) at temperature=20 °C, 30 °C and 40 °C. Cloisite® 15A shows a higher adsorption capacity at higher temperature. (Figure 9) shows the Freundlich adsorption isotherms for RB5 removal at different temperature by Cloisite 15® A.
Figure 9: Adsorption isotherms for the color removal at different temperature onto Cloisite 15® A. Freundlich isotherm model: Co=50-300mg/L, adsorbent amount (0.35g) and t=30min.
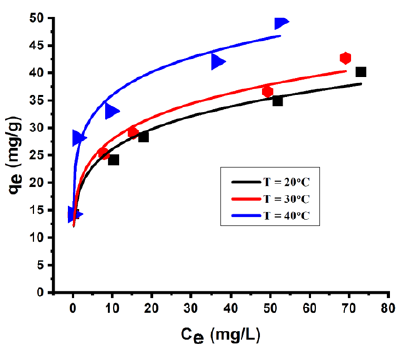
Table 2:Isotherm study of RB5 dye.
Conclusion
The present study show that Cloisite® 15A (organically modified nanoclay) is an excellent adsorbent for the removal of RB5 dye. The optimum conditions for the adsorption are adsorbent dose=0.35g, contact time=35min and pH=5. The maximum removal of RB5 dye onto Cloisite® 15A nanoclay was found to be 99.75% and 99.87% (T=35 °C and 40 °C) within 35min at C0=50mg/L. These results are better than the earlier reported results. This is primarily because in earlier studies clay has been used as an adsorbent in the micron sized particles. In the present study, the clay has been dispersed at nano scale and then used as an adsorbent. Therefore the surface area per unit mass increased tremendously as compared to using micron size clay powder. The pseudo-second-order model fits well with all experimental data with highest correlation coefficients of 0.99997 at both 30°, 35 °C, and 0.99999 at 40 °C. The adsorption equilibrium was investigated by using Langmuir, Freundlich, and Temkin isotherms. It was found that Freundlich model fit the data perfectly with the highest R2 values 0.95023, 0.95791, and 0.95177 at 20 °C, 30 °C, and 40 °C. The adsorption potential of the adsorbent is greatly affected by temperature. With a temperature rise, the Freundlich constant (KF) value increases and it illustrates that adsorption is of an endothermic type. The obtained results revealed that the Cloisite® 15A nanoclay adsorbent can be used effectively to eliminate RB5 dye.
References
- Gong R, Li M, Yang C, Sun Y, Chen J (2005) Removal of cationic dyes from aqueous solution by adsorption on peanut hull. Journal of Hazardous Materials 112(1-3): 247-250.
- Eren Z, Acar FN (2006) Adsorption of reactive black 5 from an aqueous solution: Equilibrium and kinetic studies. Desalination 194(1-3): 1-10.
- Janaki V, Vijayaraghavan K, Oh BT, Lee KJ, Muthuchelian K, et al. (2012) Starch/polyaniline nanocomposite for enhanced removal of reactive dyes from synthetic effluent. Carbohydrate Polymers 90(4): 1437-1444.
- Ip AWM, Barford JP, McKay G (2009) Reactive black dye adsorption/desorption onto different adsorbents: Effect of salt, surface chemistry, pore size and surface area. Journal of Colloid and Interface Science 337(1): 32-38.
- Choi HD, Shin MC, Kim DH, Jeon CS, Baek K (2008) Removal characteristics of reactive black 5 using surfactant-modified activated carbon. Desalination 223(1-3): 290-298.
- Karcher S, Kornmüller A, Jekel M (2001) Screening of commercial sorbents for the removal of reactive dyes. Dyes and Pigments 51(2-3): 111-125.
- Ozdemir O, Armagan B, Turan M, Çelik MS (2004) Comparison of the adsorption characteristics of azo-reactive dyes on mezoporous minerals. Dyes and Pigments 62(1): 49-60.
- Ozcan A, Oncu EM, Ozcan AS (2006) Kinetics, isotherm and thermodynamic studies of adsorption of acid blue 193 from aqueous solutions onto natural sepiolite. Colloids and Surfaces A: Physicochemical and Engineering Aspects 277(1-3): 90-97.
- Lee JW, Choi SP, Thiruvenkatachari R, Shim WG, Moon H (2006) Evaluation of the performance of adsorption and coagulation processes for the maximum removal of reactive dyes. Dyes and Pigments 69(3): 196-203.
- Elemen S, Kumbasar EPA, Yapar S (2012) Modeling the adsorption of textile dye on organoclay using an artificial neural network. Dyes and Pigments 95(1): 102-111.
- Ziane S, Bessaha F, Marouf Khelifa K, Khelifa A (2018) Single and binary adsorption of reactive black 5 and congo red on modified dolomite: Performance and mechanism. Journal of Molecular Liquids 249: 1245-1253.
- Zhang Q, Zhang T, He T, Chen Li (2014) Removal of crystal violet by clay/PNIPAm nanocomposite hydrogels with various clay contents. Applied Clay Science 90: 1-5.
- Noll KE, Gounaris V, Hou WS (1992) Adsorption technology for air and water pollution control. Chelsea, Mich: Lewis Publishers, USA, pp. 21-22.
- Hassani A, kiransan M, Soltani RDC, Khataee A, Karaca S (2015) Optimization of the adsorption of a textile dye onto nanoclay using a central composite design. Turkish Journal of Chemistry 39: 734-749.
- Mahmoudian S (2019) Removal of cationic dyes from aqueous solution using organomodified nanoclay. International Journal of Bio-Inorganic Hybrid Nanomaterials 8(2): 95-99.
- Yang Y, Han S, Fan Q, Ugbolue SC (2005) Nanoclay and modified nanoclay as sorbents for anionic, cationic and nonionic dyes. Textile Research Journal 75(8): 622-627.
- Chen YM, Tsao TM, Wang MK (2011) Removal of crystal violet and methylene blue from aqueous solution using soil nano-clays. IPCBEE 8: 252-254.
- Shen D, Fan J, Zhou W, Gao B, Yeu Q, et al. (2009) Adsorption kinetics and isotherm of anionic dyes onto organo-bentonite from single and multisolute systems. Journal of Hazard Materials 172(1): 99-107.
- Cottet L, Almeida CAP, Naidek N, Viante MF, Lopes MC, et al. (2014) Adsorption characteristics of montmorillonite clay modified with iron oxide with respect to methylene blue in aqueous media. Applied Clay Science 95: 25-31.
- Rasouli F, Aber S, Salari D, Khataee AR (2014) Optimized removal of reactive navy blue SP-BR by organo-montmorillonite based adsorbents through central composite design. Applied Clay Science 87: 228-234.
- Jamshidi A, Rafiee M, Rad MJ (2014) Adsorption behavior of reactive blue 29 dye on modified nanoclay. Trends in Applied Sciences Research 9(6): 303-311.
- Dimitry OIH, Mansour NA, Saad ALG (2016) Influence of organic modifier loading on particle dispersion of biodegradable polycaprolactone/montmorillonite nanocomposites. International Journal of Chemical and Molecular Engineering 10(2): 283-297.
- Munagapati VS, Wen JC, Pan CL, Gutha Y, Wen JH, et al. (2020) Adsorptive removal of anionic dye (reactive black 5) from aqueous solution using chemically modified banana peel powder: Kinetic, isotherm, thermodynamic, and reusability studies. International Journal of Phytoremediation 22(3): 267-278.
- Marquardt DW (1963) An algorithm for least-squares estimation of nonlinear parameters. Journal of the Society for Industrial and Applied Mathematics 11(2): 431-444.
© 2021 Mehta R. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






