- Submissions

Full Text
Polymer Science: Peer Review Journal
Novel Method for Development of Human-and Environmentally Friendly Superhydrophobic Textiles
Chung Hee Park* and Hyae Rim Hong
Department of Textiles, Merchandising and Fashion Design, Republic of Korea
*Corresponding author:Chung Hee Park, Department of Textiles, Merchandising and Fashion Design, Seoul National University, Republic of Korea
Submission: October 26, 2020;Published: February 26, 2021

ISSN: 2770-6613 Volume1 Issue4
Abstract
This article briefly describes the development of nanostructured superhydrophobic fabrics fabricated by non-chemical finishing. The developed superhydrophobic fabrics exhibit human-and environmentally friendly properties with improved breathability and non-toxicity. The newly developed fabrics also have a high potential for practical applications.
Keywords: Roughness; Surface energy; Superhydrophobicity; Thermal aging; Alkaline hydrolysis
Introduction
The surface of a lotus leaf has two roughness regimes comprising nanoscale and microscale protrusions covered with wax, which minimize the surface contact area and trap air pockets beneath liquid droplets. Cassie, Baxter, and Wenzel demonstrated in theory that the surface wettability can be enhanced by increasing the surface roughness [1,2]. According to the Cassie-Baxter model, the contact interface between the liquid and solid surfaces is in a heterogeneous state in which the air pockets are trapped in the surface-roughening structures [1]. The apparent contact angle of a liquid droplet on a solid surface increases as the amount of trapped air pockets at the solid-liquid interface increases. Water drops deposited on a superhydrophobic surface exhibit contact angles larger than 150° and shedding angles smaller than 10°; such surfaces possess self-cleaning behaviors [3].
Mini Review
Our previous laboratory studies on the development of superhydrophobic fabrics have
focused on methods using nanoparticles and fluorine-based compounds. Shim et al. [4]
developed a superhydrophobic polyester fabric that exhibited the bouncing of water droplets
deposited on the surface by developing nanoscale roughness through carbon nanotubes
(CNTs) and water repellency using a fluorine-based coating agent. Polyester fabrics without
any treatment absorbed water droplets faster when they had higher surface roughness values.
In contrast, increased contact angles for hydrophobic fabrics treated with coating agents
were obtained with increasing roughness. A fabric treated with CNTs and fluorochemicals
exhibited a water contact angle of 160° and a shedding angle of 4.4° [4]. An engineered
procedure to obtain superhydrophobic surfaces on fabrics exhibiting specific surface
energies was suggested by applying the Cassie-Baxter model to the developed fabrics [4,5].
Superhydrophobic nylon fabrics reported by Park et al. [6] were developed through the growth
of zinc oxide nanorods followed by the vapor deposition of n-dodecyltrimethoxysilane. In
experiments using these fabrics, surface nano-roughness was quantitatively associated with
hydrophobicity; the association was confirmed by the positive correlation of the estimated
solid area fraction f1 with the sliding and shedding angles [6]. These results can be applied
to optimize the degree of superhydrophobicity by controlling the nanoscale roughness of the
fabric using nanoparticles.
Surface roughness developed using inorganic particles is highly susceptible to mechanical
abrasion, leading to the easy detachment of the particles, which can potentially harm human
health. Therefore, the development of a human- and environmentally friendly fabric is required. Our superhydrophobicity research has aimed to implement
nanoscale roughness through top-down etching methods. Park et al.
[7] fabricated a superhydrophobic polyester fabric by introducing
nanoscale roughness through oxygen plasma etching followed by
vapor deposition using hexamethyldisiloxane. It was suggested
that, as the plasma radical ions bombarded the cathode plate, iron
or chromium in the stainless-steel cathode were sputtered and
co-deposited on the sample surface. The metal clusters were then
diffused to form a self-etching mask, which prevented the chemical
reaction between oxygen plasma radicals and the polyester surface.
In contrast, in the areas with no metal clusters, rapid surface
etching occurred. This difference in etching speed depending on
the position yielded anisotropic etching, resulting in nanostructure
formation on the surface. It was observed that high contact angles
promoted lower surface energy; however, nanoscale roughness was
necessary to achieve extremely low shedding angles. In addition,
when developing nanoscale roughness, superhydrophobicity was
easier to achieve on fabric than on film. This can be explained by
the inherent microscale roughness of the fabric, in which the weave
structure significantly decreased the adhesion area for water drops
and thereby facilitated superhydrophobicity [7]. Park et al. [8]
developed a superomniphobic polyester fabric with a contact angle
of at least 160° for a liquid exhibiting a surface tension of 42dyn/
cm; this was due to the fine nanopillars formed on the fabric surface
that were covered with hexamethyl disiloxane. Furthermore,
it was also due to the nanopillar tops that were wider than the
pillar bases, which suggests the presence of re-entrant structures
that could create a condition in which pocketed air can be formed
efficiently [8]. We have also reported the successful preparation
of superhydrophobic fabrics including silk [9] lyocell [10,11] and
rayon [12] by plasma etching and chemical coating.
However, the plasma treatment process has limitations in
terms of mass production. Meanwhile, surface etching by alkaline
hydrolysis is a process commonly used in the polyester fabric
industry to improve the softness, luster, moisture permeability, and
dyeability of fabrics [13]. Youn & Park [14] developed single-sided
superhydrophobic polyester fabrics utilizing alkaline hydrolysis to
obtain nanoscale roughness. Therein, the fabric was treated with
alkaline solutions and fluoropolymer foaming emulsions were used
to prepare a one-side coated fabric surface with consideration of its
moisture permeability. As a result, a superhydrophobic polyester
fabric with improved moisture permeability was achieved. Because
the moisture permeability is influenced by the air permeability
and surface wettability of the fabric, the superhydrophobic fabric
having asymmetric wettability and an increased moisture gradient
enhanced the diffusion of moisture through the fabric [14]. Thus,
the suggested optimal conditions from this report may enable the
industrial-scale manufacturing of superhydrophobic polyester
fabrics with excellent wearing comfort and self-cleaning properties.
Figure 1: SEM images of polyester filament after alkaline hydrolysis (a, c), and after alkaline hydrolysis, dyeing with C.I. Disperse Blue 56, and thermal aging (b, d).
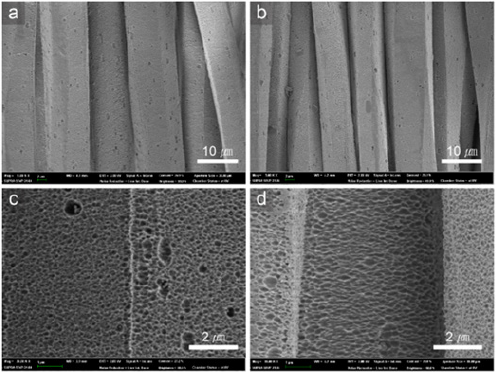
The next step in the research on superhydrophobic fabrics was to minimize or eliminate the use of chemicals for surfaceenergy reduction. Hydrophobic recovery is a phenomenon in which the contact angle of the hydrophilic surface, modified by treatments such as plasma etching, increases over time [15]. Plasma etching induces concentration differences of polar groups between the plasma-treated surface and the bulk of the material. This concentration gradient acts as the driving force, causing the reorientation of polar groups toward the bulk of the polymer from the surface because of the natural tendency to lower the surface energy. As a result of the rotational and translational motion of the polymer chains, the modified surface thereby recovers its intrinsic hydrophobicity [15]. Based on hydrophobic recovery, thermal aging is a method of recovering hydrophobicity over time by applying heat above the glass-transition temperature (Tg) to increase chain mobility and promote the rearrangement of polymer chains [16,17]. This method can reduce the surface free energy without using chemicals. Oh & Park [16] developed a superhydrophobic polyester fabric by applying an alkali treatment to obtain nanoscale roughness, followed by hydrophobization through thermal aging. We confirmed that the developed superhydrophobic fabric could repel different liquids, including water droplets, while the liquid droplets maintained spherical shapes on the surface [16]. Based on the earlier mentioned methods, Oh et al. [18] utilized a conventional dyeing process to achieve simultaneous dyeing and superhydrophobicity in polyester fabrics, because the disperse dyeing process involved a heat treatment. First, alkaline hydrolysis was applied to introduce nanoscale roughness (Figures 1a & 1c). Then, the drying process to fix the dispersed dye on the fabric and thermal aging to decrease the surface free energy were conducted simultaneously (Figures 1b & 1d). The developed polyester fabric exhibited superhydrophobicity and self-cleaning ability for several liquids (Figures 2 & 3). Furthermore, its colorfastness under washing and sunlight and its hand value were also enhanced after thermal aging [18]. Considering that clothing typically undergoes a dyeing process, the proposed process has high potential for the development of superhydrophobicity in colored fabrics through a relatively easy method. However, thermal aging is limited because it requires times and temperatures of more than 5h and 130 °C, respectively [18].
Figure 2: Bouncing behavior of water droplets(droplet volume 12.5μL) on the superhydrophobic polyester woven fabric treated with alkaline hydrolysis, dyeing with C.I. Disperse Blue 56, and then thermal aging

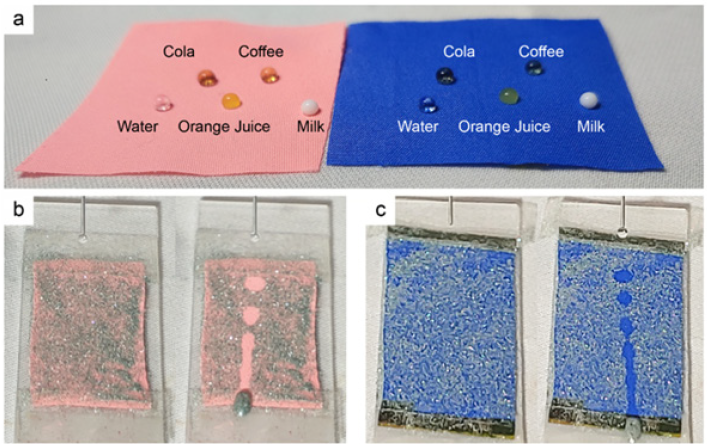
Figure 3: Repellency to various liquid droplets (a) and self-cleaning of the superhydrophobic polyester fabrics treated with alkaline hydrolysis, dyeing with C.I. Disperse Red 277 (b, left) or C.I. Disperse Blue 56 (c, right), and then thermal aging.
Kim et al. [19] compared the wetting properties of polyamide
6 (PA6) and polypropylene (PP) films after oxygen plasma
etching and hexamethyldisiloxane vapor deposition to analyze
the contributions of these surface modifications. Analyses of
the plasma-etched films over several days of aging showed that
hydrophobic recovery after oxygen plasma etching occurred
faster in the PP film than in the PA6 film. The intrinsic low surface
energy of the PP film favored the faster recovery of hydrophobicity,
because the polar groups introduced by plasma etching tended
to return to the original and stable low-surface-energy state [19]. Based on the finding that the intrinsic surface characteristics of
substrates affect the rate of hydrophobic recovery, Oh et al. [20]
analyzed the effects of molecular chain flexibility and the surface
energy of polymeric materials on the formation of nanostructures
and the recovery rate for superhydrophobicity after thermal aging.
The optimal conditions for achieving superhydrophobicity for
different materials were investigated by varying the oxygen plasma
etching durations and thermal aging times and temperatures [2].
It was shown that PP and polytetrafluoroethylene films with Tg
values below room temperature were easily hydrophobized at
lower temperatures for shorter periods compared to those needed
for polyamide or polyester films with higher Tg values. In particular,
PP in fabrics showed contact angles of approximately 180° within
2-3h of treatment. Based on these results, we concluded that the
energy consumption of the thermal aging process for PP fabrics can
be reduced. Therefore, PP with both low Tg and low surface energy
was used to prepare an energy-efficient superhydrophobic fabric
[21]. After thermal aging at 120 °C for 1h, a superhydrophobic PP
fabric was successfully obtained.
In addition to self-cleaning functions, superhydrophobic
surfaces have functional properties including antibacterial
and anti-fouling behaviors that prevent the adhesion of
microorganisms and contamination, anti-corrosion, anti-fogging,
and anti-frosting. Therefore, further research is underway to
promote superhydrophobicity in smart textiles by partially
performing the process to fabricate smart fabrics through simple
and energy-efficient fabric finishing methods [22-25]. Liquid
drops and dirt can be easily removed from the developed selfcleaning
and smart fabrics, thus preventing the degradation of
the fabrics by moisture and contamination [24]. Hong et al. [25]
developed a multifunctional polyester fabric with antibacterial,
superhydrophobic, and conductive abilities using silver and copper
nanoparticles and hydrophobic processing. Superhydrophobicity of
the fabric was achieved with the combined metal treatment followed
by hydrophobic coating, because the height difference between the
nanoparticles created dual nanostructures that formed trapped
air pockets, which minimized the contact area between the liquid
drop and the surface of the treated fabric. Moreover, the fabric
with combined metal treatment maintained conductivity even
after hydrophobic coating and exhibited a greater antibacterial
effect than the fabric with a single metal treatment [25]. This fabric
is expected to have high potential for commercial applications,
considering that viruses are threatening our daily lives.
Hong et al. [26] also investigated the influence of surface
nanostructures on the wettability of polyvinylidene fluoride
(PVDF) nanowebs by varying the surface structures through
electrospinning and carbon tetrafluoride plasma etching. The
dynamic behavior of water droplets on the surface changed from a
rose effect to a lotus effect after the introduction of fine nanoscale
roughness by plasma etching on the PVDF nanoweb; this roughness
had a hierarchical structure of microbeads and nanofibers. This is
because the three phase contact line of the PVDF nanoweb-waterair
became discontinuous, and the amount of air pockets at the interface increased when the water droplets came into contact
with the nanoweb. Thus, we confirmed that it was important to
reduce the adhesive force and the impact of negative pressure by
decreasing the contact area between the surface and the water
droplets to achieve superhydrophobicity [26]. The developed
electrospun PVDF nanoweb and the conditions of the surface
structure for special wettability (e.g., the lotus effect) derived in
this study are expected to be applicable in smart materials with
self-cleaning functions.
Conclusion
In this study, superhydrophobic fabrics were developed by fabricating nanostructures with various nanoparticles or plasma etching and by lowering the surface energy using fluorine-based or non-fluorine coating agents. The proposed new method is humanand environmentally friendly, and it can be used to fabricate nanostructures by utilizing the common alkaline hydrolysis process and to lower the surface energy by thermal aging without chemical coating. Because the use of fluorine-based or toxic chemicals for surface energy reduction should be minimized, the creation of effective surface roughness to achieve superhydrophobicity has become more important. Furthermore, the newly developed superhydrophobic fabric exhibits diverse dynamic water droplet behaviors depending on conditions such as droplet volume, falling height, and the presence of particles on the surface. These findings can be widely applied to self-cleaning textiles such as medical gowns, smart textiles, and car interior fabrics, where different dynamic behaviors and anti-soiling functions are required based on the use of the fabrics.
Acknowledgement
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (Ministry of Science and ICT; Grant Nos. NRF-2016M3A7B4910940 and NRF- 2018R1A2B6003526).
References
- Cassie ABD, Baxter S (1944) Wettability of porous surfaces. Trans Faraday Soc 40(11): 546-551.
- Wenzel RN (1936) Resistance of solid surfaces to wetting by water. Ind Eng Chem 28(8): 988-994.
- Park S, Kim J, Park CH (2015) Superhydrophobic textiles: Review of theoretical definitions, fabrication and functional evaluation. J Eng Fibers Fabr 10(4).
- Shim MH, Kim J, Park CH (2014) The effects of surface energy and roughness on the hydrophobicity of woven fabrics. Text Res J 84(12): 1268-1278.
- Shim MH, Kim J, Park CH (2015) Development of superhydrophobic fabrics by surface fluorination and formation of CNT-induced roughness. Mat Sci 21(1): 68-73.
- Park Y, Park CH, Kim J (2014) A quantitative analysis on the surface roughness and the level of hydrophobicity for superhydrophobic ZnO nanorods grown textiles. Text Res J 84(16): 1776-1788.
- Park S, Kim J, Park CH (2017) Influence of micro and nano-scale roughness on hydrophobicity of a plasma-treated woven fabric. Text Res J 87(2): 193-207.
- Park S, Kim J, Park CH (2016) Analysis of the wetting state of super-repellent fabrics with liquids of varying surface tension. RSC adv 6(51): 45884-45893.
- Oh JH, Ko TJ, Moon MW, Park CH (2014) Nanostructured superhydrophobic silk fabric fabricated using the ion beam method. RSC Adv 4(73): 38966-38973.
- Kwon SO, Park CH, Kim J (2015) Breathable, antistatic and superhydrophobic PET/lyocell fabric. J Eng Fibers Fabr 10(3).
- Kwon SO, Ko TJ, Yu E, Kim J, Moon MW, et al. (2014) Nanostructured self-cleaning lyocell fabrics with asymmetric wettability and moisture absorbency (part I). RSC Adv 4(85): 45442-45448.
- Kim HS, Park CH (2016) Effect of biaxial tensile extension on super hydrophobicity of rayon knitted fabrics. RSC Adv 6(53): 48155-48164.
- Han MS, Park Y, Park CH (2016) Development of superhydrophobic polyester fabrics using alkaline hydrolysis and coating with fluorinated polymers. Fibers and Polym 17(2): 241-247.
- Youn S, Park CH (2019) Development of breathable Janus superhydrophobic polyester fabrics using alkaline hydrolysis and blade coating. Text Res J 89(6): 959-974.
- Mortazavi M, Nosonovsky M (2012). A model for diffusion-driven hydrophobic recovery in plasma treated polymers. Appl Surf Sci 258(18): 6876-6883.
- Oh JH, Park CH (2018) Robust fluorine‐free superhydrophobic PET fabric using alkaline hydrolysis and thermal hydrophobic aging process. Macromol Mater and Eng 303(7): 1700673.
- Oh JH, Ko TJ, Moon MW, Park CH (2017) Nanostructured fabric with robust super hydrophobicity induced by a thermal hydrophobic ageing process. RSC Adv 7(41): 25597-25604.
- Oh JH, Park CH (2020) Colorful fluorine‐free superhydrophobic polyester fabric prepared via disperse dyeing process. Adv Mater Interfaces 7(10): 2000127.
- Kim J, Kim HS, Park CH (2016) Contribution of surface energy and roughness to the wettability of polyamide 6 and polypropylene film in the plasma-induced process. Text Res J 86(5): 461-471.
- Oh JH, Moon MW, Park CH (2020) Effect of crystallinity on the recovery rate of super hydrophobicity in plasma-nanostructured polymers. RSC Adv 10(18): 10939-10948.
- Kim S, Oh JH, Park CH (2020) Development of energy-efficient superhydrophobic polypropylene fabric by oxygen plasma etching and thermal aging. Polymers 12(11): 2756.
- Lee SJ, Yun C, Park CH (2019) Electrically conductive and superhydrophobic textiles via pyrrole polymerization and surface hydrophobization after alkaline hydrolysis. Text Res J 89(8): 1436-1447.
- Lee S, Park CH (2019) Conductivity, super hydrophobicity and mechanical properties of cotton fabric treated with polypyrrole by in-situ polymerization using the binary oxidants ammonium peroxodisulfate and ferric chloride. Text Res J 89(12): 2376-2394.
- Lee S, Park CH (2018) Electric heated cotton fabrics with durable conductivity and self-cleaning properties. RSC Adv 8(54): 31008-31018.
- Hong HR, Kim J, Park CH (2018) Facile fabrication of multifunctional fabrics: Use of copper and silver nanoparticles for antibacterial, superhydrophobic, conductive fabrics. RSC Adv 8(73): 41782-41794.
- Hong HR, Park CH (2020) The influence of nanostructure on the wetting transition of polyvinylidene fluoride nanoweb: from the petal effect to the lotus effect. Text Res J.
© 2021 Chung Hee Park. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






