- Submissions

Full Text
Polymer Science: Peer Review Journal
The Application Possibilities of Copper Carbon Mesocomposites as Synergists of CorrosionInhibitors and Stimulates of Protective Coating Formation
Kodolov VI1,2*, KodolovaChukhontseva VV1,2 and Reshetnikov SM3
1Basic Research-High Educational Centre of Chemical Physics & Mesoscopics, Russia
2M T Kalashnikov Izhevsk State University, Izhevsk, Russia
3Udmurt State University, Russia
*Corresponding author: Vladimir I Kodolov, Basic Research-High Educational Centre of Chemical Physics & Mesoscopics, M T Kalashnikov Izhevsk State University, Russia
Submission: February 29, 2020;Published: September 08, 2020

ISSN: 2770-6613 Volume1 Issue2
Opinion
From our recent publications [1,2] follows that the metal carbon mesoscopic composites radiate the negative charged quants within media and stimulate the media polarization. This mesocomposites energetic action on media leads to media self-organization and the density growth, and also chemical bonds formation. It is possible the high surface energy of metal carbon mesocomposites influences on the corrosion inhibitor reactivity and the adsorbed layer protective properties. According to Pletnev MA, et al. [3] the addition of Copper Carbon mesocomposite to such corrosion inhibitor as 1-morpholine methyl cyclohexyl amine is effective at the quantity equaled to 0,001mg/m3. In this paper it is shown that the joint using of Copper Carbon mesocomposite with previous corrosion inhibitor improves the protective properties in different corrosive media. The mesocomposite content increasing in the mixture with corrosion inhibitor promotes to the growth of protection degree at the corrosion. Below the dependence of the inhibitor protection degree from Copper Carbon mesocomposite concentration logarithm presents (Figure 1). The mesocomposite containing inhibitor efficacy relations in comparison with initial inhibitor are increased. These relations changes are shown in the (Figure 2). In accordance with this figure the protection degree of modified inhibitor is increased in 5,4 times in the comparison with initial inhibitor at the decreasing of its quantity from 25 to 5mg/dm3. Mesocomposite fulfills two functions: 1) for corrosion active agent decrease; 2) for the protective film creation on the material surface. These functions are carried owing to the presence on the mesocomposite carbon shell the unpaired electrons and double bonds.
Figure 1: The dependence of the inhibitor protection degree at steel corrosion from copper carbon mesocomposite concentration logarithm.
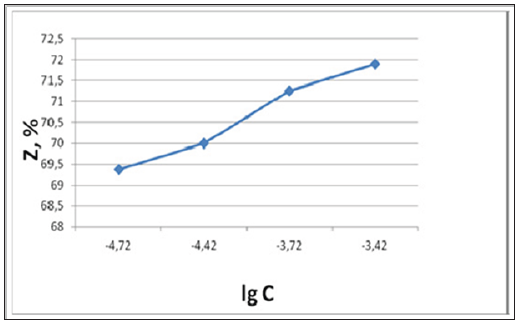
Figure 2: The dependence of the inhibitor protection degree from concentrations of inhibitor andthe mixture of inhibitorand mesocomposite. The inhibitor concentration, mg/m31: (grey)-without mesocomposite; 2: (yellow)-with mesocomposite (0,001mg/m3).
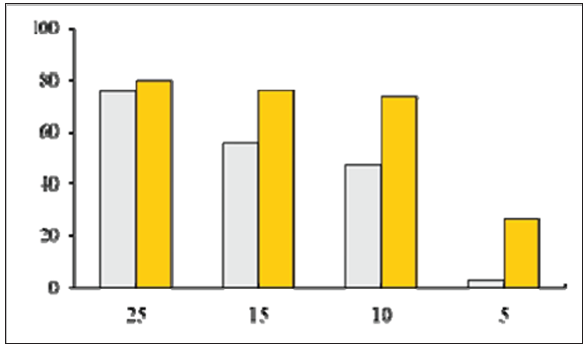
Therefore the following conclusion can be made: Cu-C mesocomposite can be effective inhibitor of corrosion. Actually the stable coating with the protection degree approximately 95% can be obtained from the oil fine suspension of the Phosphorus containing mesocomposite at the multi graded thermal processing (with the temperature increasing to 500 ˚C) at the mesocomposite quantity increasing. The effective protective coating formation is explained by the mesocomposite action as the stimulator of radical processes within hydrocarbon medium.
References
- Kodolov VI, Trineeva VV, Kovyazina OA, Vasilchenko YM (2012) Production and application of metal/carbon nanocomposites. In: The problems of nano chemistry for the creation of new materials. Torun, Poland. pp. 23-36.
- Kodolov VI, Kodolova Chukhontseva VV (2019) Fundamentals of chemical mesoscopic. M T Kalashnikov Izhevsk State Technical University, Russia. p. 218.
- Pletnev MA, Ovechkina OA, Buldakova NS (2014) The influence of metal carbon nanocomposites on protective action of corrosion inhibitors. Intellectual Systems in Engineering 1(23): 150-152.
© 2020 Vladimir I Kodolov. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






