- Submissions

Full Text
Polymer Science: Peer Review Journal
Green Technology: Time to Use Chitosan Membrane
Soontarapa K*
Department of Chemical Technology, Thailand
*Corresponding author: Soontarapa K, Department of Chemical Technology, Thailand
Submission: November 28, 2018;Published: November 30, 2018

ISSN: 2770-6613 Volume1 Issue1
Editorial
I raise this topic for the chemists and chemical engineers in the new era who expects an application of knowledge for clean and sustainable communities. Production of renewable raw materials and use of renewable feedstock is an example on the principles of sustainable development and green chemistry. Membrane based separation, considered as a substitution over traditional separation processes, has gained an important place in chemical technology and are used in a broad range of applications. A membrane is defined as a perm selective barrier interposed between two phases. It imparts separation through controlled and selective mass transfer of one of the components to be separated from one bulk phase to other. Based on the origin, membranes can be classified as natural or synthetic, neutral or charged. On the concept of a clean and sustainable future that emphasized the retrieval and reutilization of valuable products from waste streams, natural polymer chitosan is of my interest.
Chitosan is a linear polysaccharide composed of randomly distributed β-(1→4)-linked D-glucosamine (deacetylated unit) and N-acetyl-D-glucosamine (acetylated unit). It is made by treating the chitin shells of shrimp and other crustaceans with an alkaline substance, like sodium hydroxide. The amino group in chitosan has a pKa value of ~6.5, which leads to a protonation in acidic to neutral solution with a charge density dependent on pH and the %DA-value [1]. Chitosan can also be easily modified by grafting new functional groups onto the polymer backbone. The nontoxic, biodegradable and biocompatible properties of chitosan provide great potential for many applications. In this article, I would like to present an application of chitosan membrane on dehydration of esterification reaction of palm fatty acid distillate (PFAD).
PFAD is a by-product from physical refining of crude palm oil. Although it can be used, for example, to produce biofuel, candles, soaps, other oleochemical products, as well as animal feed, it is a non-desired output of the palm oil refining process. Its use does not drive palm oil production or expansion of its cultivation. Thailand is in third place in the world palm oil production ranking with 3.8% of the total global production. Currently, we have about 70 crude palm oil extraction mills with a combined production capacity around 5 million tons. On the downstream industry, we have 18 palm oil refinery plants with an annual production capacity of 2.4 million tons of which 5% are palm fatty acid distillate. 35% of all output of crude palm oil is used for cooking oil and the other 45% is used for biodiesel production. On the Alternative Energy Development Plan (2015-2036), it is expected that Thai Government will set a target output for biodiesel of 7 million liters per day by 2036 on the assumption that the average price of crude oil will be 50USD/ barrel [2].
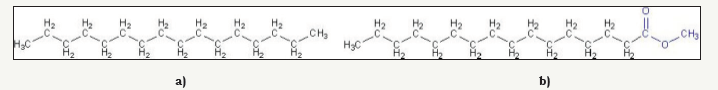
Biodiesel is an alternative renewable fuel that has gained massive attention in recent years. The combustion of biodiesel is 75% cleaner than petroleum diesel. Carbon dioxide emission is about 78% less. It produces lesser soot (particulate matter), carbon monoxsside, unburned hydrocarbons, and sulfur dioxide [3]. Biodiesel consists of long chain mono-alkyl esters and is typically made by chemically reacting of vegetable oil or animal fat with an alcohol producing fatty acid esters. Figure 1a & 1b shows the chemical structure difference between petroleum diesel and biodiesel. The petroleum diesel contains only a hydrocarbon chain but biodiesel contains both hydrocarbon chain and ester which helps in complete combustion and reduced pollution.
Figure 1:chemical structure of petroleum diesel.
Figure 1b: chemical structure of biodiesel [4].
Palm fatty acid distillate (PFAD) usually contains high free fatty acid (FFA) with the value of 87.1±1.3% in this study. Generally, the feedstock with high FFA will undergo a two steps process for biodiesel production. It needs acid catalyzed esterification step to convert most of FFA to less than 2% before alkali catalyzed transesterification step in biodiesel production. The reaction in acid catalyzed esterification step as shown in equation (1) is a reversible reaction. To keep esterification reaction forwardly as much as possible, the product water must be removed immediately. A 2L chitosan membrane reactor operating under pervaporation principle has been used in this work. On the principle of this process, the feed is in liquid form and permeate diffuses out of the membrane is in vapor form. That is there are preferential molecules absorb into the membrane, phase transformation together with diffusion from the feed side to the outlet side of the membrane, and then leaving from the membrane as a vapor.
The reactor as shown in Figure 2 consisted of 3 parts; i.e., upstream, membrane cell and downstream parts. The upstream part is for reaction with a capacity of 2L. The upstream part and downstream part are separated by a membrane cell. The downstream part is connected with a vacuum pump to create the driving force of the membrane process.
Figure 2:Membrane reactor for synthesis of PFAD biodiesel.

The chitosan membrane can be easily cast as a sheet by a phase inversion method. However, we use commercial chitosan membranes produced by a local manufacturer, SS Membrane Co Ltd., for possibly scaling in the future. Symmetric dense uncross linked and crosslinked chitosan membranes are presented in this article. Their thicknesses are in the range of 30-40μm. The hydrophilic characteristics of membranes on water uptake, pure water permeability and contact angle of uncross linked chitosan membranes are 0.97±0.02 g/g, 0.0032 L/h-m2-psi and 77.8±1.3 degree, respectively.
Those of crosslinked chitosan membranes are 0.71±0.02g/g, 0.0016L/h-m2-psi and 92.5±1.4 degree, respectively. In view of membrane performance, the uncross linked chitosan membrane was more desirable than the crosslinked one because of its higher hydrophilicity. During the reaction, we must make sure that the PFAD feedstock and biodiesel product must not be diffused through the membrane with water. On checking the membrane retention property, we found that the chitosan membranes could reject both molecules completely even using very high feed pressure of 200- 300psi comparing with atmospheric operating pressure of this reaction. It could be stated that there is no loss of either PFAD or biodiesel through the chitosan membranes. Methanol is the other important chemical for the reactions that must not be lost through the membrane.
On the measurement of methanol uptake & permeability, the values of 0.081±0.006 g/g and 0.0 L/h-m2-psi, respectively, are obtained for uncross linked chitosan membranes. Those for crosslinked chitosan membranes are 0.053±0.007 g/g and 0.0 L/h-m2- psi, respectively. Comparing with the values for water, it could be stated that only the water could diffuse through the membrane without methanol loss during the reaction (Figure 2)
The esterification reaction was fixed at 60°C with the following variations:
1. Molar ratio of PFAD: MeOH 1:6 to 1:15
2. Reaction time: 20-120min
3. H2SO4 concentration: 0.5 -2.5 wt%
4. Methanol feed rate: ss10-50mL/min
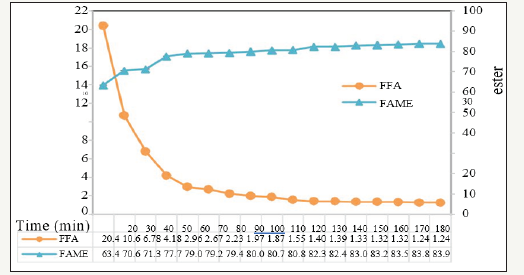
The optimum condition was at PFAD to methanol ratio of 1:15, 2.0wt% H2SO4 and 20ml/min methanol feed rate for 180min. The esterification product containing FFA and methyl ester of 1.24±0.01 and 90.7±.01wt%, respectively, were obtained. Their changes with time could be seen in Figure 3.
Figure 3:Changes of methyl ester and free fatty acid.
References
- ChitosanChitosan
- Chetchuda C (2018) Palm Oil Industry. Krungsri Research, Thailand 2018-2020.
- Biodiesel vs Diesel
- Ruhul AM, Kalam MA, Masjuki HH, Rizwanul FIM, Rehama SS, et al. (2015) State of the art of biodiesel production processes: a review of the heterogeneous catalyst. RSC Advances 5(122): 101023-101044.
© 2018 Soontarapa K. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






