- Submissions

Full Text
Perceptions in Reproductive Medicine
Endocrine Dysfunction Leads to Increase Risk of Developing Infertility in Nepalese Male Population
Nilam Thakur1,2, Nurakant Neupane2, Puja Gyawali2, Santosh Khanal3, Govardhan Joshi3, Sandeep Thapa3, Pabitra Bista3, Ajay Jang Kunwar3, Akriti Bharati4, Shalini5, Ajit Kumar Saxena5* and Amar Prakash Garg5
1Shobhit Institute of Engineering & Technology, Meerut, India
2Bir Hospital, Kathmandu, Nepal
3Nova International Diagnostics, Kathmandu Nepal
4Vatsalya IVF Center, Kathmandu, Nepal
5All India Institute of Medical Sciences, Patna, India
*Corresponding author:Ajit Kumar Saxena, Department of Pathology/Lab Medicine, All India Institute of Medical Sciences, Patna, India
Submission: March 16, 2024;Published: May 27, 2024

ISSN: 2640-9666Volume6 Issue2
Abstract
Present study includes hormonal profile and semen analysis in two clinically diagnosed male infertile azoospermic and oligospermic cases. Male infertility, affecting 15%-20% of couples globally and there a need for comprehensive assessment to explore the etiopathology of disease. A cross-sectional study involving (n=100) individuals having age group 24 to 50 selected for hormonal profiles such as Follicle Stimulating Hormone (FSH), Luteinizing Hormone (LH), and Testosterone using an immunoassay. Findings reveals, increased level of FSH and LH levels but decreased testosterone level in azoospermic cases, suggesting, hormonal imbalances may lead to affect spermatogenesis. Significant variations and correlation analyses in azoospermic and oligospermic revealed intricate relationships between age, hormone levels, and semen analysis outcomes, enriching our understanding of male reproductive health complexities.
Keywords:Male infertility; Hormonal assay; Azoospermia; Oligospermia; Spermatogenesis
Introduction
Infertility is usually described as the failure to conceive after at least a year of regular unprotected intercourse and 15%-20% of couples are affected by reproductive disorder [1]. Globally, estimated 72.4 million people are affected and WHO estimates that 9% of couples worldwide struggle with fertility issues. In 50% of couples are solely responsibility 30% in male and 20% in female factor [2]. Etiopathology of male infertility is a complex phenomenon affecting approximately 7% of the global human population with wide range of heterogeneous phenotypes, from congenital or acquired urogenital reproductive disorders, endocrine dysfunction including immunological factors during spermatogenesis [3]. Epidemiological data available over the past twenty years revealed that about 30% of cases shows abnormal pathology, therefore, the male factor is at least partly responsible in about 50% of infertile couples [4].
At present includes physical examination, semen analysis, biochemical examination, hormonal assay, immunological, radiological investigations, cytogenetic and testicular biopsy [5]. Gonadotrophins (FSH, LH) and testosterone are the prime regulators of germ cell development. The successful and complete male germ cell development depends on the balanced endocrine interplay of hypothalamus, pituitary and the testis. Episodic secretion of Gonadotrophin Releasing Hormone (GnRh) by the hypothalamus elicits the pulsatile release of gonadotrophins i.e. FSH and LH [6,7]. These hormones play an important role in steroidogenesis for male gonads. In the testes, LH is the exclusive steroidogenic hormone acting for the regulation of interstitial cells of Leydig, and FSH acts for Sertoli cells. FSH binds with receptors in the Sertoli cells and stimulate spermatogenesis. LH stimulates the production of testosterone in Leydig cells and Sertoli cells for differentiation of peritubular cells of the seminiferous tubules regulating spermatogenesis [7]. Testosterone can be metabolized in peripheral tissue to the potent androgen in the form of dihydrotestosterone and act as the potent estrogen. These androgens and estrogens act independently to modulate the activity of LH secretion [5]. Isolated rise in FSH is an important and sensitive biomarker for the differentiation of the germinal epithelium. The increased FSH level in men with azoospermia or severe oligozoospermia (< 5million sperm/ml) is indicator a symptomatic damage of seminiferous tubule [8]. The failure of pituitary to secret FSH and LH will result in disruption of testicular function followed by hypogonadotropic hypogonadism leads to decreased sperm counts [9].
Materials and Methods
The analytical cross-sectional study aimed to investigate male infertility, specifically focusing on azoospermic and oligospermic cases in comparison to age-matched normozoospermia act as controls. A total of (n=100) individuals aged between 24 to 50 years, that comprise 50 case samples suspected with male infertility (azoospermic and oligospermic primary infertile) and equal number of control subjects (males of proven fertility) were recruited for the study. Moreover, the female partner of the respective individual male subject was not having any causes related to infertility. Total (5.0 ml) of venous blood was collected from each subject, following the acquisition of informed consent and collection of relevant information. Quantitative analysis of hormone profiles, including Follicle-Stimulating Hormone (FSH), Luteinizing Hormone (LH), and Testosterone was performed using Immunoassay analyzer (Access 2 Immunoassay Analyzer, Beckman Coulter, Inc. USA).
Result
In this study, the distribution of azoospermia, oligospermia, and those with normozoospermia act as controls were studied according to age groups. The majority of azoospermia cases fall within the 30-40 age group, whereas, controls are more evenly distributed across the 30-40 and 40-50 age groups (Table 1). Oligospermia cases are observed across all age groups, mostly in the 30-40 age group. In the study, individuals with azoospermia exhibited significantly elevated levels of mean FSH (17.94±14.14), mean LH (9.92±7.42), and lower mean testosterone (3.54±1.63) compared to the control group (mean FSH: 5.85±2.41, mean LH: 5.42±2.74, mean testosterone: 5.35±2.05), while those with oligospermia demonstrated intermediate values (mean FSH: 6.95±4.88, mean LH: 4.85±2.33, mean testosterone: 3.96±1.79) (Figure 1). The median and Interquartile Range (IQR) of Hormonal Levels (FSH, LH, and Testosterone) based on semen analysis reveal notable differences between azoospermia, oligospermia, and control groups. Azoospermia cases exhibit higher FSH levels as compared to controls, indicating a potential dysfunction in spermatogenesis (Table 2).
Table 1:Age wise distribution of semen analysis in different groups.
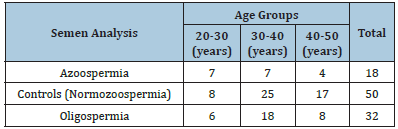
Figure 1:Bar diagram representing mean value of hormone levels based on semen analysis
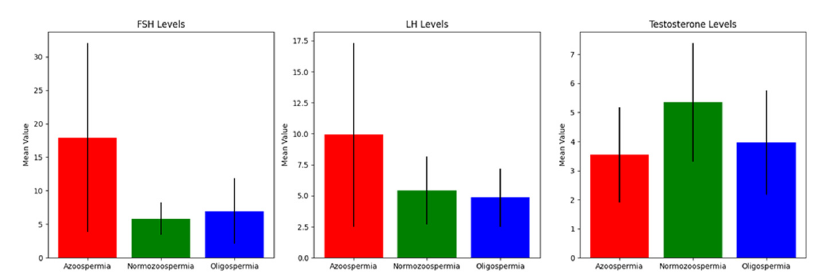
Table 2:Statistical analysis showing significant association between hormone profile and semen analysis.
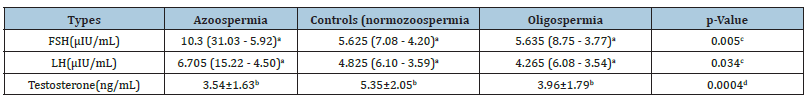
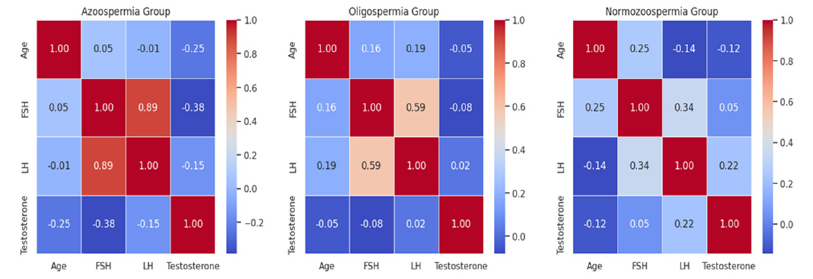
Furthermore, the Kruskal-Wallis test demonstrates statistically significant differences in FSH and LH levels among azoospermia, oligospermia, and control groups. This suggests that hormonal variations are associated with different semen analysis outcomes. One-way ANOVA analysis of testosterone levels also revealed statistically significant differences. In the azoospermia group, there is a negative correlation was observed between age and testosterone, while FSH and LH show positive correlations with each other and a negative correlation with testosterone. In the oligospermia group, age shows a positive correlation with FSH and LH, and negative correlation with testosterone. In the control group, age is positively correlated with FSH and negatively correlated with testosterone, while FSH and LH show a positive correlation with each other and with testosterone (Figure 2).
Figure 2:Correlation between different variables such as azoospermia, oligospermia and control (normozoospermia).
Discussion
Present study aimed to investigate endocrinological variations in male infertility within Nepalese population in azoospermic and oligospermic cases and compared with to agematched normozoospermia individuals act controls with confirm fertility. The results reveal several key findings that contribute to the understanding of male reproductive health. The age-wise distribution of infertility cases demonstrated a higher prevalence of azoospermia in the 30-40 age group, while normozoospermia cases were more evenly distributed across the 30-40 and 40-50 age range. In oligospermia cases, on the other hand, were observed across all age groups, with a majority in the 30-40 age group. This agespecific distribution underscores the importance of considering age-related factors when evaluating in male infertility.
The incidence of primary endocrine defects in sub-fertile men is less than 3% and is rare in men with sperm concentrations greater than 5 million per ml [10]. This study assessed the endocrine environment in relation to spermatogenesis and sperm maturation. The serum FSH, LH and testosterone levels were estimated in infertile azoospermic, oligospermic males and age matched normozoospermic fertile cases (controls). The mean FSH and LH levels had significant rise in azoospermic males in contrast to oligospermic and normozoospermic males. The findings of this study had significantly elevated gonadotropins (FSH and LH) levels in azoospermic males than that of ologospermic and normozoospermic in accordance with the earlier studies [10-16] with mean LH and FSH levels, 9.92mIU/mL and 17.94mIU/mL in azoospermic males were also in concordance.
The higher FSH have been reported to be associated with human subfertility by different studies [14,17,18]. Various studies have also highlighted elevated serum FSH levels are associated with azoospermia [18-20]. The abnormal spermatogenesis may have a normal serum FSH, but a marked elevation in serum FSH is clearly indicative of abnormality in spermatogenesis [21]. In this study, the median and Interquartile Range (IQR) of hormonal levels (FSH, LH, and Testosterone) based on semen analysis reveal notable differences between azoospermia, oligospermia, and normozoospermia (control) groups. Azoospermia cases exhibit higher FSH levels compared to normozoospermia cases, indicating a potential endocrine dysfunction during spermatogenesis. It has been reported that, in addition to FSH secreting pituitary adenoma or testicular failure, hyperactivity of the FSH axis, could also be due to mutations of FSH receptor gene [22].
The data from the Kruskal-Wallis test and one-way ANOVA also confirmed statistically significant differences in FSH, LH, and testosterone levels among azoospermia, oligospermia, and normozoospermia groups. These variations suggest that hormonal dysregulation is associated with different semen analysis outcomes, supporting the evidence that endocrine disorders contribute significantly to male infertility [23-25]. The correlation analysis revealed intriguing patterns in different semen analysis groups. In the azoospermia group, a negative correlation was observed between age and testosterone, while FSH and LH showed positive correlations with each other and negative correlations with testosterone. In the oligospermia group, age exhibited a positive correlation with FSH and LH, while testosterone showed a negative correlation with age. In the normozoospermic group, age positively correlated with FSH and negatively correlated with testosterone, and FSH and LH exhibited positive correlations with each other and with testosterone. These correlations highlight the complexity of the interplay between age, hormonal levels, and semen analysis outcomes, providing valuable insights for further research [26,27]. The variation in the hormone profile and semen analysis may be either due to genetic heterogeneity of folate metabolism regulatory methylene tetrahydrofolate C677T gene polymorphism in oligozospermic cases or genetic factor that increase “risk factor” for developing infertility in male [28].
Conclusion
The findings conclude the highlight the relevance of hormonal analysis in understanding male reproductive health. The elevated FSH and LH levels appear to be associated with azoospermia, emphasizing the potential role of hormonal dysfunction leads to abnormal spermatogenesis. These results contribute valuable insights knowledge of complexity between age, hormonal levels, and semen analysis outcomes, providing a foundation for further research in male fertility. The important component of evaluating male infertility is to quantifying the hormones of the hypothalamuspituitary- gonadal axis. A patient’s abnormalities in the production of certain hormones, such as FSH, LH, and Testosterone, can be used as a diagnostic biomarker to determine the etiopathology of their azoospermia/oligospermia patients. These findings have implications for clinical practice, emphasizing the importance of incorporating hormonal analysis alongside traditional semen analysis in the evaluation of male infertility. The identified correlations between age, hormonal levels, and semen analysis outcomes pave the way for further research in the field of male reproductive health. Understanding these complex relationships is crucial for developing targeted interventions and personalized treatment strategies for individuals having male infertility. The limitations of this study include its cross-sectional design, sample size and the need for further longitudinal research to validate the observed associations. Additionally, exploring the cytogenetic aspects of infertility could provide a more comprehensive understanding of the underlying mechanisms. Future studies should aim to elucidate the genetic and environmental factors influencing endocrinological and cytogenetic variations in male infertility.
References
- Katz DJ, Teloken P, Shoshany O (2017) Male infertility-the other side of the equation. Aust Fam Physician 46 (9): 641-646.
- Boivin J, Bunting L, Collins JA, Nygren KG (2007) International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Human Reproduction 22(6): 1506-1512.
- Krausz C (2011) Male infertility: Pathogenesis and clinical diagnosis. Best Pract Res Clin Endocrinol Metab 25(2): 271-285.
- Shaban SF (2007) Male infertility overview: assessment, diagnosis and treatment. IVF com, Atlanta USA.
- Naznin L, Parveen T, Parvin M, Giti S, Ahmed A, et al. (2022) FSH, LH and testosterone status and categorization of infertile azoospermic males-a single centre cross sectional study. Bangladesh J 2(1): 28-33.
- De Kretser DM (1979) Endocrinology of male infertility. Br Med Bull 35(2): 187-192.
- Donnell L, Lachlan RI, Wreford NG, Robertson DM (1994) Testosterone promotes the conversion of round spermatids between stages VII and VIII of the rat spermatogenic cycle. Endocrinology 135(6): 2608-2614.
- Bergmann M, Behre HM, Nieschlag E (1994) Serum FSH and testicular morphology in male infertility. Clin Endocrinol (Oxf) 40(1): 133-136.
- Zhang JR, Yao B, Wang YM, Cui YX, Wang SK, et al. (2003) Detection of sexual hormone in semen of patients with idiopathic azoospermia or oligospermia and its significance. Zhonghua Nan Ke Xue 9(4): 279-281.
- Martindu Pan R (2009) Endocrine pathology: Effects on male fertility reproductive health. Geneva Foundation for Medical Education and Research.
- Sultan C, Craste B, Audran F, Iqbal Y, Ville C (1985) Hormonal evaluation in male infertility, Annales De Biologie Clinique, pp. 63-6.
- Zabul J, Mierzejewski W, Rogoza A (1994) Usefulness of examining gonadotropin hormones and testosterone in men with abnormal semen. Ginekol Pol 65(2): 71-74.
- Weinbauer GF, Nieschlag E (1995) Gonadotrophin control of testicular germ cell development. Tissue Renin-Angiotensin Systems: Current Concepts of Local Regulators in Reproductive and Endocrine Organs 377: 55-65.
- Subhan F, Tahir F, Ahmad R, Khan ZD (1995) Oligospermia and its relation with hormonal profile. J Pak Med Assoc 45(9) :246-247.
- Babu SR, Sadhnani MD, Swarna M, Padmavathi P, Reddy PP (2004) Evaluation of FSH, LH and testosterone levels in different subgroups of infertile males. Indian Journal of Clinical Biochemistry 19(1): 45-49.
- Al Daghistani HI, Abdel Dayem M (2002) Hyperprolactinemia and hypergonadotropins in infertile males with severe oligospermia and azoospermia. Internet J Endocrinol 3: 1540-2606.
- Subhan F (2000) Seminal and hormonal profiles of fertile and sub-fertile Pakistani men. Pak J Med Res 39(1): 42-45.
- Ovesen P, Jørgensen JL, Kjær T, KKY Ho, Ørskov H, et al. (1996) Impaired growth hormone secretion and increased growth hormone-binding protein levels in sub fertile males. Fertil Steril 65(1): 165-169.
- Perraguin JS, Audebert A, Emperaire JC, Parneix I (1997) Ongoing pregnancies after intracytoplasmic injection using cryopreserved testicular spermatozoa. Hum Reprod 12(12): 2706-2709.
- Subhan F, Tahir F, Ahmad R, Khan ZU (1995) The study of azoospermic patients in relation to their hormonal profile (LH, FSH and Testosterone). Rawal Med J 22(6): 25-27.
- The Male Infertility Best Practice Policy Committee of the American Urological Association (2006) Report on optimal evaluation of the infertile male. Fertil Steril 86(5): S202-S209.
- Weinbauer GF, Luetjens CM, Simoni M, Nieschlag E (2010) Physiology of testicular function. In: Nieschlag E, Behre HM, Nieschlag S (Eds), Andrology: Male Reproductive Health and Dysfunction, Berlin, Heidelberg: Springer Berlin Heidelberg, pp. 11-59.
- Jungwirth A, Giwercman A, Tournaye H, Diemer T, Kopa Z, et al. (2012) European association of urology guidelines on male infertility: The 2012 update. Eur Urol 62(2): 324-332.
- Bhasin S, Pencina M, Jasuja GK, Travison TG, Coviello A, et al. (2011) Reference ranges for testosterone in men generated using liquid chromatography tandem mass spectrometry in a community-based sample of healthy nonobese young men in the Framingham Heart Study and applied to three geographically distinct cohorts. J Clin Endocrinol Metab 96(8): 2430-2439.
- Layman L (2002) Human gene mutations causing infertility. J Med Genet 39(3): 153-161.
- Krausz C, Escamilla A (2018) Genetics of male infertility. Nat Rev Urol 15(6): 369-384.
- Foresta C, Bettella A, Garolla A, Ambrosini G, Ferlin A (2005) Treatment of male idiopathic infertility with recombinant human follicle-stimulating hormone: a prospective, controlled, randomized clinical study. Fertil Steril 84(3): 654-661.
- Saxena AK, Agarwal M, Kumar A, Singh CK (2020) Genetic heterogenicity of MTHFR C677T allele modulate hormonal dysfunction associated risk factor in the case of male infertility. Int J Dev Res 10(12): 42700-42705.
© 2024 Ajit Kumar Saxena. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






