- Submissions

Full Text
Perceptions in Reproductive Medicine
A Clinical Case of Successful Correction of Autoimmune Progesterone Dermatitis Using a Drospirenone-Containing Oral Contraceptive
Makatsaria Alexander1 and Ekaterina Slukhanchuk2*
1Department of Obstetrics and Gynecology of Institute of Children’s Health, Academician of the Russian Academy of Sciences, Russia
2Department of Obstetrics and Gynecology, Filatov Clinical Institute of Children’s Health, Sechenov University, Sechenov University, Russia
*Corresponding author:Ekaterina Slukhanchuk, Department of Obstetrics and Gynecology, Filatov Clinical Institute of Children’s Health, Sechenov University, Sechenov University, Russia
Submission: July 28, 2022;Published: January 12, 2023

ISSN: 2640-9666Volume5 Issue3
Summary
Autoimmune Progesterone Dermatitis (APD), a disease with an incompletely defined epidemiology and pathogenesis, is increasingly reminiscent of itself. The most common triggers for the development of the disease are pregnancy, taking progesterone and combined oral contraceptives. The situation is aggravated by the fact that there are no definite criteria for its diagnosis, there is no understanding of what specialists should deal with these patients, and, ultimately, what effective therapy should be. In this article, we present a clinical case of APD in a young patient, which developed without any triggers before the implementation of the reproductive function, which impaired the quality of life. The oral contraceptive containing drospirenone, selected for the patient, made it possible to save her from the clinical manifestations of APD for a long period of time. Autoimmune progesterone dermatitis is a disease that occurs predominantly in women of reproductive age. At the same time, in the case of a severe course of the disease, therapy reaches the extirpation of the uterus with appendages and subsequent estrogen monotherapy, which is unacceptable in patients who have not yet realized their reproductive function. It is necessary to continue research in the field of the pathogenesis of this condition, as well as the search for possible methods of therapy and correction of symptoms.
Keywords: Autoimmune progesterone dermatitis; Progesterone; Drospirenone; Allergic reaction
Background
Definition
Autoimmune Progesterone Dermatitis (APD) is a rare disease with premenstrual exacerbations associated with hypersensitivity to progesterone. Autoimmune dermatitis associated with hypersensitivity to endogenous estrogens has also been described [1,2].
History reference
For the first time, a case of cyclic rash, which could be caused by an allergy to endogenous sex hormones, was reported by Geber in 1921. The patient described by him suffered from urticaria, which could be caused by an injection of autologous blood serum taken before menstruation [3]. The concept of hypersensitivity to sex hormones was further developed in 1945 when Zondek and Bromberg described several patients with skin lesions (including cyclic urticaria) associated with menstruation and menopause. They found in these patients a delayed-type allergic reaction to intradermal progesterone and clinical improvement after desensitizing therapy. Patients in the control group did not respond to intradermal administration of progesterone [4]. In 1951 Guy et al. reported a patient with premenstrual urticaria. With intradermal administration of corpus luteum extracts, she experienced a pronounced allergic reaction. The patient was later successfully treated with desensitization therapy. The term “autoimmune progesterone dermatitis” was proposed by Shelly et al. in 1964, who for the first time demonstrated the partial effect of estrogen therapy and cure after oophorectomy [5].
Pathogenesis
The mechanism of a woman’s sensitization to her own progesterone is not clear. According to one of the most common hypotheses, taking drugs containing progesterone contributes to sensitization to endogenous progesterone [6]. Synthetic progesterone is thought to be antigenic enough to produce antibodies that then cross-react with natural progesterone and elicit an immune response in the premenstrual period. However, not all women with APD take synthetic progestogens. In some patients, the disease first manifests during pregnancy, in some during the first years after menarche without the use of synthetic progestogens [7].
Clinical manifestations
APD manifests itself as a cyclic exacerbation of symptoms. Symptoms are resistant or practically resistant to conventional therapy, but ovulation suppression drugs usually work well. Usually, the time of onset of symptoms coincides with the postovulatory increase in plasma progesterone [8]. A few days after the onset of menstruation, the concentration of progesterone decreases, and the severity of clinical manifestations also decreases. Thus, dermatitis can begin 7 days before menstruation and end 1-3 days after the end of menstruation. Manifestations are often resistant to conventional therapy. The manifestations of APD include eczema, polymorphic exudative erythema, dyshidrosis, urticaria, stomatitis, rash as in dermatitis herpetiformis [9,10].
Diagnostics
The markers of the disease are the relationship with the premenstrual period and the menstrual cycle, the disappearance of symptoms with the suppression of ovulation, positive tests with progesterone. An intradermal test with synthetic progesterone usually causes an utricle rash as a manifestation of an immediatetype reaction, but a delayed-type allergic reaction is also possible. Despite the frequent use of an intradermal progesterone test, its results are unreliable, since progesterone is insoluble in water, and all solvents have a pronounced irritant property. Skin reactions at the injection site of progesterone are often difficult to interpret, and false positive results are possible. In addition, skin necrosis often develops at the injection site, epithelializing with the formation of a scar. However, a persistent delayed reaction at the injection site indicates an increased sensitivity to progesterone. It is also possible to use intramuscular and oral progesterone (using dydrogesterone) tests in the first half of the menstrual cycle, when the manifestations of APD are minimal. In doubtful cases, but with a pronounced clinical picture, therapy with GtrH (Gonadotropin Releasing Hormone) agonists is carried out for 6 months, and, against its background, an oral progesterone test is used, obtaining increased sensitivity [11]. The onset or exacerbation of skin diseases often coincides with pregnancy, which is associated with an increase in the concentration of progesterone and estrogen. However, there are reports of patients in whom the manifestations of APD spontaneously resolved during pregnancy. It is known that during pregnancy in many patients with allergic diseases, the condition improves. This suggests that increased cortisol secretion during pregnancy reduces immune reactivity. It is also possible that a gradual increase in the concentration of hormones in some patients has a desensitizing effect [12].
Treatment
In mild forms of the course of the disease, local glucocorticoid ointments and creams and antihistamines are effective in therapy. With symptoms of moderate severity and severe - glucocorticoids orally and parenterally. In some cases, the use of Danazol (200mg 1-2 days before the expected onset of menstruation and withdrawal after 3 days) [13] courses, as well as Tamoxifen (20mg daily) [14] and GtrH agonists [15,16] is effective. Desensitization is a specific hypo sensitization, which is carried out according to different schemes. The time for its implementation ranges from several days to several weeks, and sometimes months. The method consists in stimulating the immune system with increasing doses of the allergen, while blocking IgE and reducing their effect on mast cells. Such therapy usually lasts for 6 months for several years. Effective desensitization schemes for APD are currently not proposed for use in clinical practice. Ovariectomy is certainly an extreme method used in severe and refractory to other therapy types of the disease [5]. The same extreme methods, but with the possibility of estrogen monotherapy for young women, include extirpation of the uterus with appendages and subsequent estrogen monotherapy with proven hypersensitivity to progesterone and the ineffectiveness of other methods of therapy [5].
Once inside the cell cytoplasm, cisplatin works by displacing chlorine ligands by hydroxyl or aquo groups, giving rise to the corresponding aquo and hydroxy complexes (Figure 2). Once these aquo and hydroxocomplexes have been formed, cisplatin has become a potent electrophile capable of binding to the thiol groups of proteins, to the N-dadors of nucleic acids, and specifically to the purine bases such as adenine and guanine. As mentioned above, due to the different side effects of cisplatin, research continued on this type of compound in an attempt to reduce it without losing its efficacy. This led to the second generation of platinum compounds, consisting of oxaliplatin [3] and carboplatin [4-7]; (Figure 3). All the compounds discussed so far are based on platinum’s oxidation number two, but there are also compounds in oxidation number four. One of these is cis, cis, trans-[Pt(NH3)2Cl2(O2CC6H5)2] (Figure 4), which has been used inside silk fibroin nanoparticles, because this makes it easier for the compound to reach the inside of tumours. It is inside tumours that the compound exerts its greatest cytotoxic activity. As reported in the article published by Lozano Pérez A et al. [8]. Based on all the research that has been done on the cytotoxic activity of platinum compounds, it was natural that the same research would be done, but with varying metals in the different compounds.
Clinical Case
Patient 25 years old
Anamnesis
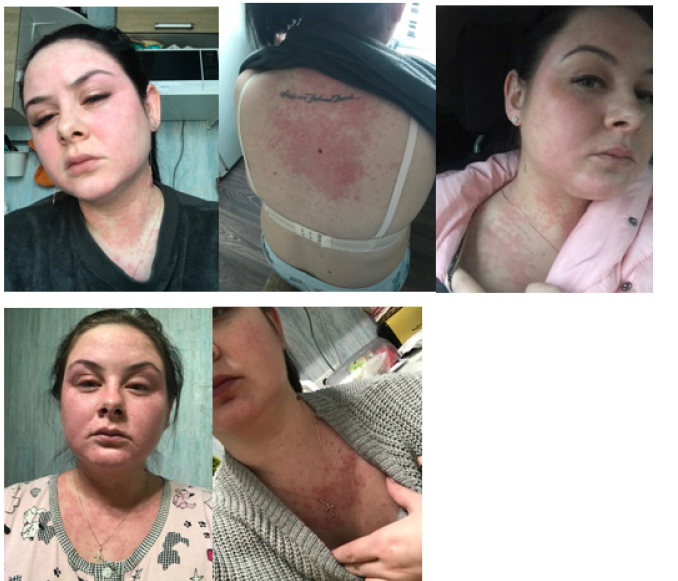
She was born as a healthy full-term baby girl. She grew and developed normally. Menarche at the age of 12, menstruation was established within 3 years. After the establishment of the menstrual cycle (28 days), she began to notice the appearance of a rash, redness on her arms, legs, back and face, resembling urticaria (Figure 1). At first, the cyclicity of what is happening is what the patient did not appreciate. She has taken place inspection at the dermatologist, the immunologist and the allergist. Over time, the severity of symptoms increased. Several episodes of Quincke’s edema. In the age period from 16 to 20 years, she notes hospitalizations with severe manifestations of anaphylaxis 2-3 times a year. Conducting standard allergological tests did not reveal any triggers. However, the cyclic nature of the rashes was noted, while with a regular cycle, the manifestations of symptoms were monthly, and with delays of menstruation were absent. In this regard, the patient was referred for a consultation with a gynecologist-endocrinologist. When conducting a blood test for hormones, a slight increase in the concentration of testosterone in the blood was revealed, with which periods of irregular menstruation were associated. The patient was prescribed a combined oral contraceptive with an antiandrogenic effect, containing cyproterone acetate, both for the purpose of contraception and for the purpose of regulating the menstrual cycle. Against the background of taking the drug, allergy symptoms became more frequent, lost their cyclical nature, increased and required more frequent use of glucocorticoids. The drug was cancelled.
Figure 1:Manifestations of APD before contacting the clinic.
Diagnostics
The patient came to our clinic. The diagnosis was established
clinically. The basis for the clinical diagnosis of APD was:
A. Cyclical changes during periods of a regular menstrual
cycle.
B. The onset of symptoms at the age of onset of ovulatory
cycles.
C. The development of symptoms from the 19th-20th day of
the menstrual cycle at the 28th day cycle and their completion on
the 2nd-5th day of the menstrual cycle.
D. The constant nature of the manifestation of the disease
against the background of COCs containing cyproterone acetate
(progesterone derivative), since in this monophasic preparation
each of the 21 tablets contains 2mg of cyproterone acetate and
0.35mg of ethinyl estradiol.
This diagnosis was confirmed by the development of severe symptoms in response to the use of dydrogesterone 10mg 2 times a day in the first phase of the cycle for 2 days with an increase in symptoms, and therefore taking the drug as an oral test was completed.
Therapy
Taking into account the young age, the need for contraception, as well as periodic menstrual cycle failures against the background of increased testosterone concentration, after additional examination and the exclusion of contraindications, the patient was offered a monophasic oral contraceptive containing a spironolactone derivative-drospirenone 3mg and 0.02mg ethanol estradiol. When prescribing the drug, we counted on its effectiveness due to the fact that the drug does not contain a component with a molecular structure similar to progesterone, as well as on a decrease in the production of endogenous progesterone during the use of the drug.
Dynamics
The use of the drug for a long time provided the patient with a long-term remission of APD and reliable contraception. For 4 years she took an oral contraceptive. There were no symptoms of APD, which allowed her to complete her studies without interruptions in hospitalization and start her career. The patient is currently planning a pregnancy. The drug has been cancelled. Simultaneously with the withdrawal of the drug, the presence of ovulation is assessed, ovulation for three consecutive cycles is present according to ultrasound and ovulation tests. The further course of the disease in the event of pregnancy and its progression requires observation.
Discussion
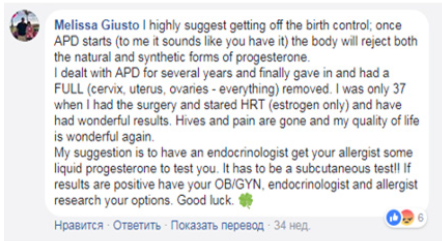
Autoimmune progesterone dermatitis is a fairly common disease, the exact prevalence of which has not been sufficiently studied due to the lack of awareness of gynecologists, dermatologists and immunologists-allergists-specialists who are the first to turn to patients, as well as the lack of available methods to confirm the presence of this disease. The prevalence of the disease and the lack of success in its treatment are reported by numerous international groups on social networks, where patients themselves share their symptoms, types and effectiveness of therapy recommended to them by doctors from different countries (Figure 2). Thus, an analysis of the Autoimmune Progesterone Dermatitis group on Facebook showed that in the majority of registered patient users, symptoms first appeared during pregnancy or after it, in a smaller number of patients while taking progesterone preparations and combined oral contraceptives. The most effective method of radical treatment is surgical treatment-the uterus extirpation with appendages and subsequent hormonal estrogen monotherapy (Figure 3).
Figure 2:Pages of the social network Facebook dedicated to APD.
Figure 3:Facebook “Autoimmune progesterone dermatitis”.
However, this method is certainly extremely radical, it cannot be offered to patients in all countries, it is not paid by insurance companies, given the lack of indications for surgery for such a diagnosis. The American biologist Gregory Goodwin Pincus is considered the father of the birth control pill. Based on a discovery in the 1930s that injections of the female sex hormone, progesterone, suppressed ovulation in animals, he decided to use this hormone in contraceptive pills for women. He also proposed the idea of using a combination of two synthetic female sex hormones, estrogen, and progestogen, in one tablet. Any oral combined contraceptive contains two components: ethinyl estradiol and a synthetic progestogen. Both components are involved in the suppression of ovulation, which is the main mechanism of contraceptive action. Synthetic progestogens differ significantly in the spectrum of pharmacological effects and, consequently, in their influence on metabolic processes and tolerability.
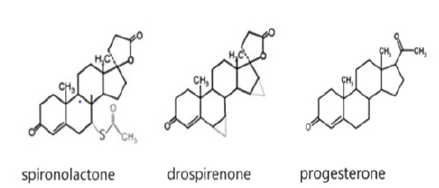
The only common property of progestogens that unites them into one pharmacological group is their ability to interact with progestogen receptors and have a progestogen effect. Drospirenone (chemical name: 6b, 7b, 15b, 16b-dimethylene-3-oxy-17a-pregn- 4-ene-21, 17-carbolactone) is a progestogen, a derivative of 17a-spirolactone, with the pharmacodynamic properties of natural progesterone (Figure 4). Ovulation suppression is achieved with the use of drospirenone at a daily dose of 3mg. The need for such a dose is explained by the absence of an ethynyl radical in the drospirenone molecule and, as a result, a milder effect on progesterone receptors compared to progestogens containing an ethynyl radical, which are dosed in micrograms per day. The absence of an ethynyl radical in the drospirenone molecule increases its safety, as it excludes the inhibition of cytochrome P-450 in the liver, which is characteristic of ethylated progestogens. Perhaps it was the molecular structural differences between progesterone and drospirenone that made it possible to avoid the immune response when using the proposed oral contraceptive./
Figure 4:Molecular structure of progesterone, spironactone and drospirenone.
Conclusion
The problem of APD is widespread among women of reproductive age in different countries. Its statistics are difficult. Diagnostics is not carried out due to the lack of diagnostic algorithms in most medical organizations, as well as test systems for laboratory diagnostics and skin allergy tests. Also, the principles of therapy have not been developed, especially in patients who have not realized their reproductive function. The use of oral contraceptives in such patients is considered unacceptable, despite the fact that the composition of oral contraceptives is fundamentally different precisely in the progestin component, and there are drugs that include progestins with a different molecular structure, which means they are unable to provoke APD symptoms. In addition, given the pathogenesis of the condition and the fact that in some patients the onset of the disease begins precisely with the use of COCs, it makes sense to think that drugs containing progestins with a molecular structure different from progesterone can be used as a prevention of the early onset of APD before the first pregnancy and childbirth.
References
- Mutasim DF, Baumbach JL (2003) Bullous autoimmune estrogen dermatitis. Journal of the American Academy of Dermatology 49(1): 130-132.
- Miura T, Matsuda M, Yanbe H, Sugiyama S (1989) Two cases of autoimmune progesterone dermatitis. Immunohistochemical and serological studies. Acta Derm Venereol 69(4): 308-310.
- Oskay T, Kutluay L, Kaptanocglu A, Karabacak O (2002) Autoimmune progesterone dermatitis. European Journal of Dermatology 12: 589-591.
- Meggs WJ, Pescovitz OH, Metcalfe D, Loriaux DL, Cutler G, et al. (1984) Progesterone sensitivity as a cause of recurrent anaphylaxis. New England Journal of Medicine 311(19): 1236-1238.
- Shelley WB, Preucel RW, Spoont SS (1964) Autoimmune progesterone dermatitis: cure by oophorectomy. Jama 190: 35-38.
- Stephens C, Black M (1989) Perimenstrual eruptions: autoimmune progesterone dermatitis. Semin Dermatol 8(1): 26-29.
- Snyder JL, Krishnaswamy G (2003) Autoimmune progesterone dermatitis and its manifestation as anaphylaxis: a case report and literature review. Ann Allergy Asthma Immunol 90(5): 469-477.
- Vasconcelos C, Xavier P, Vieira A, Martinho M, Rodrigues J, et al. (2000) Autoimmune progesterone urticaria. Gynecol Endocrinol 14(4): 245-247.
- Herzberg AJ, Strohmeyer CR, Cirillo Hyland VA (1995) Autoimmune progesterone dermatitis. J Am Acad Dermatol 32(2): 335-338.
- Moghadam BK, Hersini S, Barker BF (1998) Autoimmune progesterone dermatitis and stomatitis. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontology 85(5): 537-541.
- Halevy S, Cohen AD, Lunenfeld E, Grossman N (2002) Autoimmune progesterone dermatitis manifested as erythema annulare centrifugum: confirmation of progesterone sensitivity by in vitro interferon-γ Journal of the American Academy of Dermatology 47(2): 311-313.
- Warin A (2001) Case 2. Clinical and Experimental Dermatology 26(1): 107-108.
- Shahar E, Bergman R, Pollack S (1997) Autoimmune progesterone dermatitis: effective prophylactic treatment with danazol. International Journal of Dermatology 36(9): 708-711.
- Stephens C, Wojnarowska F, Wilkinson J (1989) Autoimmune progesterone dermatitis responding to tamoxifen. British Journal of Dermatology 121(1): 135-137.
- Slater JE, Raphael G, Cutler GB, Loriaux DL, Meggs WJ, et al. (1987) Recurrent anaphylaxis in menstruating women: treatment with a luteinizing hormone-releasing hormone agonist-a preliminary report. Obstetrics and Gynecology 70(4): 542-546.
- Yee K, Cunliffe W (1994) Progesterone‐induced urticaria: response to buserelin. Br J Dermatol 130(1): 121-123.
© 2023 Ekaterina Slukhanchuk. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






