- Submissions

Full Text
Perceptions in Reproductive Medicine
Some Anticancer Agents: From Cisplatin to Half Sandwich Ruthenium(II) And Iridium(III) Compounds
Gabriel García-Sánchez*
Department of Inorganic Chemistry, Faculty of Chemistry, International Campus of Excellence, Campus Mare Nostrum, University of Murcia, Spain
*Corresponding author: Gabriel García- Sánchez, Department of Inorganic Chemistry, Faculty of Chemistry, International Campus of Excellence, Campus Mare Nostrum, University of Murcia, Spain
Submission: December 20, 2022;Published: January 04, 2023

ISSN: 2640-9666Volume5 Issue3
Platinum
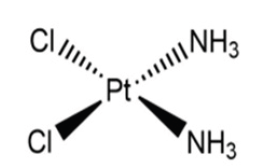
The first major discovery in the fight against cancer, in the field of metallo pharmaceuticals, was in 1965; the synthesis of cis-diaminochloroplatinum(II) or as it is commonly known, cisplatin (Figure 1). Rosenberg B [1] was studying the effect of electric fields on cell growth. He constructed a system with two platinum electrodes through which an electric current was passed over a population of Escherichia coli ( E. coli) and found that they stopped dividing, but Ribonucleic Acid (RNA) and protein synthesis did continue [1]. Cisplatin can be used for the treatment of different types of cancer, whether breast, ovarian, brain, etc. It can be used alone or in combination with other types of drugs. Despite all its benefits, it also has a number of disadvantages that have led to further progress in the research of this type of compounds in order to remedy them. Some of the side effects of this compound include cardiotoxicity, nephrotoxicity, allergic reactions and vomiting [2].
Figure 1:Chemical structure of cisplatin.
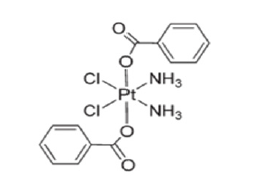
Once inside the cell cytoplasm, cisplatin works by displacing chlorine ligands by hydroxyl or aquo groups, giving rise to the corresponding aquo and hydroxy complexes (Figure 2). Once these aquo and hydroxocomplexes have been formed, cisplatin has become a potent electrophile capable of binding to the thiol groups of proteins, to the N-dadors of nucleic acids, and specifically to the purine bases such as adenine and guanine. As mentioned above, due to the different side effects of cisplatin, research continued on this type of compound in an attempt to reduce it without losing its efficacy. This led to the second generation of platinum compounds, consisting of oxaliplatin [3] and carboplatin [4-7]; (Figure 3). All the compounds discussed so far are based on platinum’s oxidation number two, but there are also compounds in oxidation number four. One of these is cis, cis, trans-[Pt(NH3)2Cl2(O2CC6H5)2] (Figure 4), which has been used inside silk fibroin nanoparticles, because this makes it easier for the compound to reach the inside of tumours. It is inside tumours that the compound exerts its greatest cytotoxic activity. As reported in the article published by Lozano Pérez A et al. [8]. Based on all the research that has been done on the cytotoxic activity of platinum compounds, it was natural that the same research would be done, but with varying metals in the different compounds.
Figure 2:Formation of aqueous and hydroxy complexes from cisplatin.
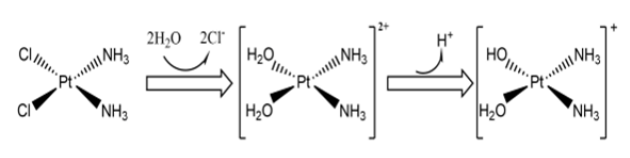
Figure 3:Oxalic platinum and carbo platinum.
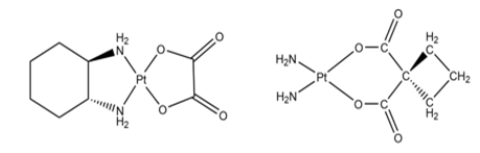
Figure 4:Chemical structure of the cis, cis, trans -[Pt(NH3)2Cl2(O2CC6H5)2].
Ruthenium
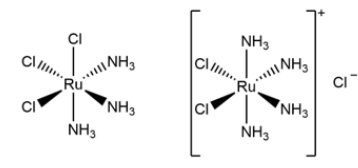
Figure 5:Structures of fac-[RuCl3(NH3)3] and cis- [RuCl2(NH3)4]Cl.
Ruthenium will be one of the metals we will study here, so we
will focus on it, as well as on iridium. After the success of platinum
compounds as anti tumour agents, it seems logical that research has
continued on different types of metals to maximize their cytotoxic
activity and to reduce their side effects. This led to ruthenium, a
metal which, as part of certain metallo pharmaceuticals, has been
shown to have greater anticancer activity and lower toxicity [9].
The functioning of these ruthenium compounds involves direct
attack on tumour cells or by inhibiting specific enzymes essential to
the cells [10]. During early research into the potential anti tumour
character of ruthenium as well as platinum, the compounds fac-
[RuCl3(NH3)3] and cis-[RuCl2(NH3)4]Cl (Figure 5) were tested on E.
coli as well as cisplatin and found to have a similar effect [11]. Upon
discovery of the effect of these ruthenium complexes, his research
continued, leading to three major families of ruthenium complexes
with anti tumour action. These three groups are:
i. “Kepler-type” complexes
ii. complexes including the ligand DMSO
iii. “semi-sandwich-type” complexes.
The “Kepler-type” complexes are those whose structure is as follows trans-[RuCl4L2], where L can be either the imidazole ligand or the indazole ligand, i.e. N-dador ligands. Among these types of ligands, the best known are: NAMI-A (a), which contains the imidazole ligand, KP1019 (b) and NKP-1339 (c), whose ligand is the indazole and differ in the counterion they have, KP1019 has the indazole cation and NKP-1319, they have a sodium (Figure 6).
Figure 6:Structures of (a) NAMI-A, (b) KP1019 and (c) NKP-1339.

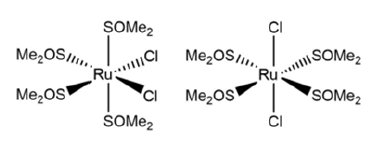
These three complexes have shown good results in different cytotoxic studies. NAMI-A is highly effective against metastasis and has a very low toxicity, being the only ruthenium complex found in the market. currently in clinical development [12], while KP1019 and NKP-1319 have been found to be very effective against tumour cells that had developed resistance to cisplatin, especially for colon cancer [13]. The next group of ruthenium complexes are those with Dimethyl Sulfoxide (DMSO) as ligand. This ligand is of great importance because it is ambidentate, as it can be coordinated to ruthenium by both the oxygen atom and the sulfur atom, and even by the double bond between the two, as if it were an olefin. Whether DMSO binds in one way or another depends on a number of electronic and steric factors [14]. Among the ruthenium complexes containing DMSO as a ligand, the cis- and trans-isomers of the [RuCl2(DMSO-S)4] complex are noteworthy (Figure 7) and the position they occupy will influence the effect they have on tumour cells. The trans-isomer is twenty times more effective than the cis- 16 isomer.
Figure 7:cis- and trans-isomers of the complex [RuCl2(DMSO-S)4].
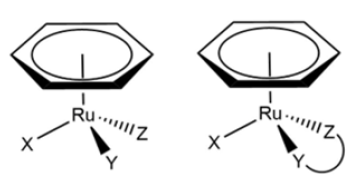
And the last group are the “half-sandwich” ruthenium complexes, which are composed of η6-arene ligands. These ligands stabilize the +2 oxidation state of ruthenium and, since this was discovered, it has been tested whether they also possess cytotoxic character like the other two groups. This type of compound has a generic structure [(η6-arene) Ru(X)(Y)(Z)] (Figure 8), the arene that is complex can vary and the three remaining ligands can be substituted by an ambidentate ligand to give the complex greater stability [15]. These ruthenium complexes act in the same way as cisplatin, they lose one of their ligands and are able to react with the target molecules. However, unlike platinum complexes, they do not act on DNA, but maintain their good cytotoxicity.
Figure 8:Ruthenium half-sandwich complexes without and with ambidentate ligand.
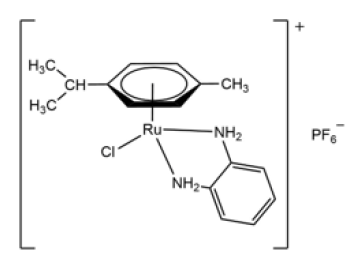
Among these compounds, those synthesized by Garcia G, et al. [15] and collaborators in 1994 with the following general formulae [(η6-arene)RuCl2L], or [(η6-C6H6)RuClL2]Cl should be highlighted. The arenes used were benzene and p-cymene, and the “L “s were potentially bidentate ligands. Of particular note is the compound [(η6-p-cymene)RuCl(o-fen)]PF6 (Figure 9); [16] , whose strong cytotoxic activity against various types of cancer led it to be used in a patent in the USA [17]. After all that has been said about ruthenium, we cannot forget other uses of ruthenium such as immunosuppressant or treatment against malaria [18].
Figure 9:
Iridium
The other metal around which this mini reviews revolves is iridium. This metal, like ruthenium and others such as osmium and rhodium, belongs to the group of precious metals, as does platinum, since they share a large number of properties, among which are their high resistance to corrosion and their low reactivity [19]. It is also used in electronics for its luminescent properties and given that it can form compounds with the same geometry as cisplatin, planar-square, when its oxidation state is +1, its use as a potential anti-tumour compound began to be studied. As research on these compounds continued, it was found that when iridium was in the +3 oxidation state, it was more “stable” than in its +1 state, and that its coordination number increased to six22. The complexes containing the pentamethylcyclopentadienyl (Cp*) ligand forming the [Ir(η5-Cp*)] fragment were the most important. These complexes containing the Cp* ligand are of the “semi-sandwich” type, as mentioned above with ruthenium. These complexes use the Cp* or variations with phenyl, biphenyl or aliphatic chain substituents. The general structure these compounds usually have is [(η5-Cp*)Ir(L^L’)Z]0/+ (Figure 10). Ligands with the ability to act as a chelating agent (L^L’) are often used to give the compound greater stability, and the other ligand is usually chloride or pyridine, as these facilitate the substitution reactions (Figure 11); [18].
Figure 10:Generic structure of iridium half-sandwich compounds with structure [(η5-Cp*)Ir(L^L’)Z]0/+.
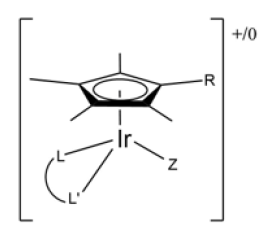
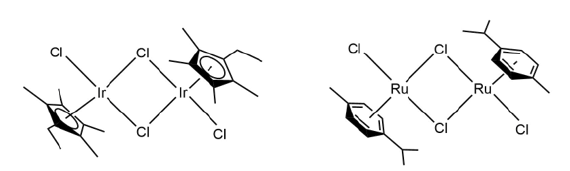
Figure 11:Structures of [Ir(η5-C5 EtMe4)Cl2]2 and [Ru(η6-p-cymene)Cl2]2.
The cytotoxic activity of iridium compounds varies to a great extent depending on the arene substituents, since, as Sadler PJ et al. [19] have observed, it is not the same when the arene is pentamethylcyclopentadienyl (Cp*) or phenyl tetra methyl cyclopentadienyl (Cpxph ) or biphenyl tetra methylcyclopentadienyl (Cpxbiph) and the bidentate ligands X^Y [19].
References
- Rosenberg B (1971) Some biological effects of platinum compounds: New agents for the control of tumors. Platinum Metals Review 15(2): 42-51.
- Dasari S, Tchounwou PB (2014) Cisplatin in cancer therapy: Molecular mechanisms of action. Eur J Pharmacol 740: 364-378.
- Boulikas T, Vougiouka M (2003) Cisplatin and platinum drugs at the molecular level. Onlogy Reports 10(6): 1663-1682.
- Kelland L (2007) The resurgence of platinum-based cancer chemotherapy. Nature Reviews Cancer 7(8): 573-584.
- Chaney SG, Campbell SL, Basset E, Wu Y (2005) Recognitio and processing of cisplatin and oxaliplatin- DNA adducts. Crit Rev Oncol Hematol 53(1): 3-11.
- Graham J, Mushin M, Kirkpatric P (2004) Oxalilplatinum. Nature Reviews Drug Discovery 3: 11-12.
- Wang D, Lippard SJ (2005) Cellular processing of platinum anticancer drugs. Nature Reviews Drug Discovery 4(4): 307-320.
- Lozano Pérez A, Abel Gil Ana L, Pérez Sergio A, Cutillas N, Hajo M, et al. (2015) Anti-tumor properties of platinum(iv) prodrug-loaded silk fibroin nanoparticles. Dalton Trans 44: 13513-13521.
- Hartinger CG, Dyson PJ (2009) Bio organometallic chemistry-from teaching paradigms to medicinal applications. Chem Soc Rev 38(2): 391-401.
- Dabroviak JC (2012) Metals in medicine. Angew Chem Int Ed Engl 38(11): 1512-1531.
- Meier-Menches SM, Gerner C, Berger W, Hartinger CG, Keppler BK (2018) Structure-activity relationships for ruthenium and osmium anticancer agents-towards clinical development. Chemical Society Reviews 47(3): 909-928.
- Yan YK, Melchart M, Habtemariam A, Sadler PJ (2005) Organometallic chemistry, biology and medicine: Ruthenium arene anticancer complexes. Chem Commun 38: 4764-4776.
- Iida J, Bell-Loncella ET, Purazo ML, Lu Y, Dorchak J, et al. (2016) Inhibition of cancer cell growth by ruthenium complexes. J Transl Med 14(1): 48.
- Alessio E, Mestroni G, Nardin G, Attia WM, Calligaris M (1988) Cis and trans-dihalotetrakis (dimethyl sulfoxide) ruthenium (II) complexes (RuX2(DMSO)4; X = Cl, Br): synthesis, structure, and antitumor activity. Inorg Chem 27(23): 4099-4106.
- García G, Solano I, Sánchez G, Santana MD, López G (1994) Organomet. Chem 467: 119-126.
- Habtemariam A, Sadler PJ (2009) Ruthenium compounds, Patent US7241913B2, United States Pat, pp. 1652-1657.
- Hartinger CG, Dyson PJ (2009) Bioorganometallic chemistry from teaching paradigms to medicinal applications. Chem Soc Rev 38(2): 391-401.
- Lu L, Liu LJ, Chao WC, Zhong HJ, Wang M, et al. (2015) Identification of an iridium (III) complex with anti-bacterial and anti-cancer activity. Scientific reports 5(1): 1-9.
- Liu Z, Sadler PJ (2014) Organoiridium complexes: Anticancer agents and catalysts. Accounts of Chemical Research 47(4): 1174-1185.
© 2023 Gabriel García-Sánchez. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






