- Submissions

Full Text
Perceptions in Reproductive Medicine
Impact of Lactobacillus Plantarum on Reproductive Potential of Male Mice Challenged with Sperm Agglutinating Escherichia Coli
Priyanka B, Chauhan A and Vijay Prabha*
Department of Microbiology, India
*Corresponding author:Vijay Prabha, Department of Microbiology, India
Submission: May 18, 2022;Published: June 14, 2022

ISSN: 2640-9666Volume5 Issue2
Abstract
TNowadays, substantial attention is being directed towards using probiotics for treating many diseases. However, the probable role of probiotics in alleviating infertility problems did not receive much attention. Keeping this in mind, the present study was carried out to determine the capability of L. plantarum 2621 to ameliorate the uropathogenic colonization and detrimental effects induced by sperm agglutinating Escherichia coli in a male murine model. For this, male Balb/c mice were divided into 3 groups viz. group I (PBS); group II (104/108 of E. coli); group III (104/108 of L. plantarum 2621) and tissue somatic indices, bacterial load, seminal parameters (sperm count, motility and viability), tissue histology and MDA levels were evaluated to assess the impact of different doses of individual bacteria. The administration of E. coli (104/108cfu) alone affected all the parameters negatively irrespective of dose, while in case of L. plantarum (104/108cfu), results obtained were comparable to PBS. After this, the combined effect of E. coli and L. plantarum 2621 was evaluated and experiments were further carried out to check the ability of L. plantarum (108cfu) to ameliorate the negative impact induced by E. coli (104/108cfu). For this, male mice were divided in to 2 groups viz. group I (PBS) and group II. Group II was further subdivided in to 2 subgroups viz. subgroup I (104cfu of E. coli + 108 of L. plantarum 2621) and subgroup II (108cfu of E. coli + 108 of L. plantarum 2621). Group I showed no significant change in any of the parameters, while in case of Subgroup I of group II, complete amelioration of the negative impact was observed in all the parameters as compared to subgroup II of group II where the negative impact of E. coli was observed.
Introduction
Probiotics are food supplements in the form of live microorganisms when consumed, impart health benefits to the host. Bacterial genera most commonly used in the probiotic preparation are Lactobacillus, Bifidobacterium, Enterococcus, Bacillus and yeasts. Based on many experimental studies and scientific observations, it has been proposed that probiotics can control both digestive and non-digestive diseases [1]. In several cases, urogenital infections are responsible for infertility in both males and females. In females, these infections occur due to the absence of Lactobacillus, which is the main component of the normal flora of the vagina. The probiotic Lactobacilli produce an anti-inflammatory response against pathogenic microorganisms. Hence, it can be hypothesized that inflammation induced infertility can be treated by the use of Lactobacillus [2]. The composition and organization of commensal bacterial communities in seminal fluids ascertain the potential causes of male infertility. The consequential inflammation compromises spermatogenesis and sperm cell function due to infection in the male reproductive tract. Sperm abnormalities like aberrant motility, deficient mitochondrial function and DNA integrity loss, are linked with the microorganisms such as Escherichia coli, Enterococcus faecalis, Neisseria gonorrhoeae, Chlamydia trachomatis, Ureaplasmaurealyticum, Candida. albicans, Trichomonas vaginalis and Mycoplasma hominis, which are found in the male urogenital tract. However, the most abundant bacteria in the seminal fluid of normal males include Staphylococcus, Anaerococcus, Corynebacterium, Lactobacillus, Prevotella, Streptococcus, Finegoldia and others [3]. It can be speculated from these observations that Lactobacillus is not only a major component of the microflora of the female vagina but is also related to the normal semen fluid in males such that the seminal parameters are affected by its presence.
Dardmeh et al. [4] have studied the effect of probiotic L. rhamnosus on sperm motility and kinematics and found an increase in the fraction of motile sperm compared to immotile sperms when probiotics are administered. Therefore, in the present study, sperm agglutinating Escherichia coli previously isolated in our laboratory and the standard strain of Lactobacillus plantarum (2621) were used to colonize in the mouse in order to study their effect on reproductive potential individually. Further, a study was undertaken to determine the capability of L. plantarum to overcome the uropathogenic colonization and reduced reproductive potential induced by sperm agglutinating E. coli in the male murine model.
Materials and Methods
Experimental animals
In the present study, sexually mature, 5-6 weeks old Balb/c males weighing 25±5g were used, which were obtained from Central Animal House, Panjab University. Standard laboratory conditions were maintained for keeping animals, with a photoperiod of 12h of light & 12h of darkness. A standard pellet diet consisting of 20- 21% crude protein, 4% fat, 5.0-7.5% crude fibre, 8-9% ash, 1.0- 1.5% calcium, 0.6-0.8% phosphorus and 50% nitrogen free extract (M/s Ashirwad Industries Pvt. Ltd.) and water ad libitum were given to all mice. All the experimental protocols were reviewed and approved by the Institutional Animals Ethics Committee of the Panjab University, Chandigarh (Approval No: PU/45/99/CPCSEA/ IAEC/2018/221) and were performed in accordance with the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India.
Microorganisms
A clinical isolate of Escherichia coli (isolated in our laboratory from semen of males undergoing analysis at PGIMER, Chandigarh) causing 100% sperm agglutination in vitro and a standard strain of Lactobacillus plantarum (MTCC 2621), were used in the present study. The standard strain was procured from Microbial Type Culture Collection, Institute of Microbial Technology, Sector 39, Chandigarh, India.
Preparation of inoculum
The sperm agglutinating E. coli and L. plantarum were grown in Luria-Bertani broth (LB) and De Man, Rogosa and Sharpe broth (MRSB), respectively, under shaking conditions for 24h at 37 ᵒC. The culture broths were centrifuged after incubation at 10,000rpm for 10min. The pellet so obtained was washed twice with Phosphate Buffer Saline (PBS) (50mM, pH 7.2). The final concentration of 104 and 108CFU/20μl of each organism was achieved by suspending the pellet in PBS buffer.
Impact of intravas deferens inoculation of E. coli/L. plantarum on reproductive potential of male mice
In order to assess the role of E. coli/L. plantarum on the
reproductive potential of male mice, Balb/c mice were divided into
three groups (I, II, III):
1. Group I (n = 3): PBS
2. Group II was further subdivided into 2 subgroups:
a) Subgroup E1 (n = 3): 104cfu/20μl of E. coli
b) Subgroup E2 (n = 3): 108cfu/20μl of E. coli
3. Group III was also further subdivided into 2 subgroups:
a) Subgroup L1 (n = 3): 104cfu/20μl of L. plantarum
b) Subgroup L2 (n = 3): 108cfu/20μl of L. plantarum
Mice were anesthetized (ketamine (75mg/kg) and xylazine (121mg/kg)) and the right vas, testis and epididymis were exteriorized aseptically via a vertical incision in the scrotum. By using 27-gauge needle, 20μl of inoculum (single dose) was instilled into the lumen of right vas deferens, towards the direction of epididymis. 3-0 silk suture was used to close incision and animals were housed individually in propylene cages to avoid transmission of organisms. No mortality due to surgical procedure was observed and the animals revived quickly.
Tissue somatic indices TSI (%)
To evaluate any effect on TSI, mice from each group were sacrificed on day 7and TSI (percent organ weight in relation to body weight) was calculated according to equation [5].
Bacterial load
The viable bacterial load was calculated after weighing the organs which were collected under sterile conditions. The organs were immersed in separate Eppendorf’s in 500μl PBS (50mM, pH 7.2). These organs were homogenized manually to form a dense mixture. 100μl of this mixture was spread on LA and MRSA plates and incubated at 37 ᵒC for 24h. The number of colonies were counted after 24h and log CFU/g of tissue was calculated.
Confirmation of reisolated microorganisms
The obtained bacterial isolates were streaked on Eosin Methylene Blue (EMB) agar plates. The Group I administered with PBS was culture negative whereas Group II administered with different doses (104/108) of E. coli confirmed the presence of E. coli by giving green metallic sheen in all the Subgroups (E1and E2). The obtained bacterial isolates were streaked on MRS agar plates. The Group I administered with PBS was culture negative whereas in Group III administered with different doses of (104/108) L. plantarum confirmed its presence by showing growth when streaked on MRS agar plates.
Analysis of Seminal Parameters
Total sperm count: After inoculation, on day 7 mice were sacrificed by cervical dislocation and with the help of dissecting kit they were dissected and abdomen was cut open. The vas deferens was pulled out and placed in freshly prepared normal saline (250μl) in glass plate. The spermatozoa were enabled to swim into the freshly prepared normal saline by gentle agitation and teasing of vas deferens. Clean glass slide was prepared with 10μl of sample with the help of micropipette and covered with cover slip (22mm x 22mm). While placing the cover slip, trapping and formation of air bubbles was avoided. For stabilization, preparation was left undisturbed for approximately 1min. It was then viewed under light microscope (Olympus India Pvt. Ltd.) at 400X magnification. Eight fields were scanned and mean number of spermatozoa in the fields was multiplied by 106.
Sperm motility and viability: A fixed volume of 10μl of the sample obtained was delivered on a clean glass slide with a micropipette, covered by a cover slip (22mm x 22mm) and examined various fields under light microscope at 400X magnification. On the basis of motility, spermatozoa were classified as either motile or non-motile. The relative percentage of motile and immotile sperms was determined after assessing different microscopic fields [6]. For viability, above procedure was repeated with spermatozoa along with eosin dye to differentiate between live and dead spermatozoa. Percentage of viable sperm was evaluated.
Tissue histology: Reproductive organs (vas deferens, cauda and testis) of mice from all groups were examined for any histopathological changes by using standard procedure, after organs fixed with 10% formaldehyde for 24hr. the paraffin embedded tissues were sectioned and stained with hematoxylin and eosin. Any significant changes in the reproductive organs were observed under 100 X and 400 X magnifications.
DA estimation: 2ml of TBA-TCA reagent (15% TCA and 0.375% TBA) was added to 100-500μl of tissue homogenates and mixed. The reaction mixture was kept for 20min in boiling water bath. Protein precipitates settled at the bottom after centrifugation at 3500g for 10min and pink color supernatant was collected. Absorbance was read at 532nm. The MDA levels were calculated in nanomoles per gram of tissue by using extinction coefficient of 1.56 x 105mol-1 (OD X Total volume/ EC X sample volume).
Combined effect of intra vas deferens inoculation of E. coli and L. plantarum on reproductive potential of male mice: To determine the role of E. coli and L. plantarum on the reproductive potential of male mice when administered in combination via intra vas deferens route, male Balb/c mice were divided into two Test Groups. Test Group I was administered with PBS while Test Group II was further divided in to two subgroups (I and II): a) Subgroup I (n = 3): 104 CFU of E. coli+ 108 CFU of L. plantarum/20μl b) Subgroup II: (n = 3): 108 CFU of E. coli+ 108 CFU of L. plantarum/20μl
After inoculation 3 mice from each group were sacrificed on day 7 and TSI (%), bacterial load, seminal parameters, histopathological changes and MDA levels were evaluated as described in section 2.4.
Result
Impact of intra vas deferens inoculation of E. coli/L. plantarum on reproductive potential of male mice
Tissue somatic indices TSI (%): In case of mice administered with PBS (Group I), the TSI (%) levels of reproductive organs of right side viz. vas deferens, cauda and testis were estimated to be 0.025±0.001, 0.051±0.0025 and 0.51±0.02 and the corresponding values in left side were 0.06±0.002, 0.039±0.001 and 0.522±0.001, respectively
In Subgroup E1 (104) of Group II, the TSI (%) of reproductive organs on right side were 0.023±0.002, 0.03±0.0017 and 0.417±0.0015 whereas on left, the corresponding values were 0.021±0.0025, 0.041±0.002 and 0.506±0.002, respectively. In Subgroup E2 (108), the TSI (%) of reproductive organs on right side were 0.031±0.0026, 0.031±0.003 and 0.349±0.002 whereas on left, the corresponding values were 0.023±0.003, 0.039±0.002 and 0.484±0.002, respectively. From the above results, it was observed that Subgroup E2 showed significant changes in TSI (%) in right side of reproductive organs whereas in left side no significant changes were observed in E1 as compared to Group I (PBS).
In Subgroup L1 (104) of Group III, the TSI (%) of reproductive organs on right side were 0.023±0.002, 0.051±0.003 and 0.505±0.002 whereas on left, the corresponding values were 0.061±0.002, 0.038±0.002 and 0.516±0.002, respectively. In Subgroup L2 (108), the TSI (%) of reproductive organs on right side were 0.0256±0.001, 0.0513±0.0015 and 0.499±0.002 whereas on left side, the corresponding values were 0.061±0.0025, 0.037±0.0015 and 0.511±0.001, respectively. From the above results, it was observed that no significant changes in TSI (%) in right side and left side were observed in both the subgroups of Group III (L. plantarum) as compared to Group I (PBS).
Bacterial load: On day 7, when the homogenates of reproductive organs viz. vas deferens, cauda and testis were plated on LA plate, the results showed that the homogenates of reproductive organs of Group I (PBS) demonstrated no viable count. On the other hand, when the various reproductive organs of mice challenged with E. coli (Group II) and L. plantarum (Group III) were plated on LA plates on day 7 bacterial load were observed in all the reproductive organs (Table 1) (Figure 1 & 2). The bacterial count was present in both sides of reproductive organs in the subgroups of Group and II and III indicating that bacteria could evade to left side, however, with higher bacterial load in right side as compared to left side.
Table 1: Enumeration of bacterial load (in terms of log10cfu/g of tissue) from vas deferens, cauda and testis of mice challenged intravasally with E. coli/L. plantarum on day 7.
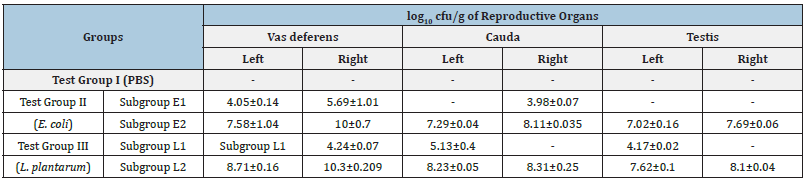
- No viable count, [Values represent Mean±SD]
Figure 1: Representative photographs of bacterial load from the homogenates of mice administered with i) PBS (a-c) {a) Left/Right vas deferens, b) Left/Right cauda, c) Left/Right testis}; ii) 104/108cfu/20μl of E. coli (d-i) {d) Left vas deferens, e) Left cauda, f) Left testis, g) Right vas deferens, h) Right cauda, i) Right testis} on day 7.
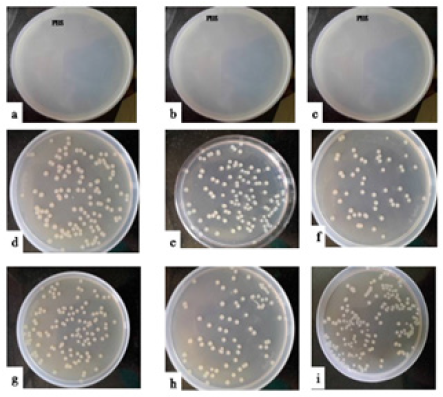
Figure 2:Representative photographs of bacterial load from the homogenates of mice administered with i) PBS (a-c) {a) Left/Right vas deferens, b) Left/Right cauda, c) Left/Right testis}; ii) 104/108cfu/20μl of L. plantarum (d-i) {d) Left vas deferens, e) Left cauda, f) Left testis, g) Right vas deferens, h) Right cauda, i) Right testis} on day 7.
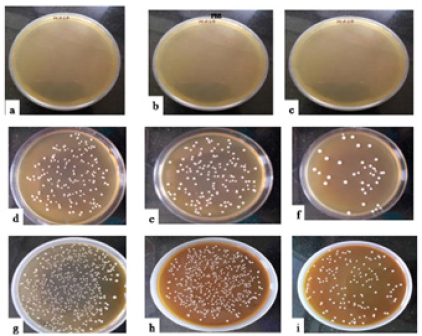
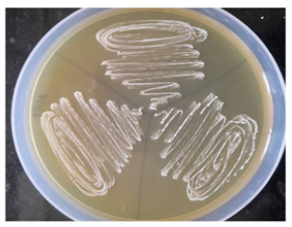
Reisolation of administered microorganisms: The obtained bacterial isolates were streaked on Eosin Methylene Blue (EMB) and Lactobacilluson MRS agar plates. E. coli was confirmed by the presence of green metallic sheen on EMB agar and L. plantarum was confirmed by its growth on MRS agar (Figure 3 & 4).
Figure 3: Representative photographs of E. coli of Group II showing growth on EMB plates on day 7.
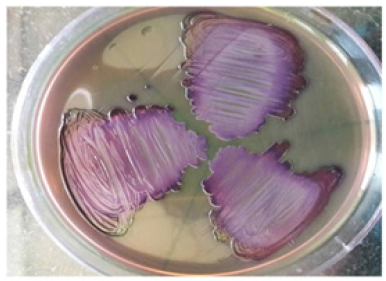
Figure 4:Representative photographs of L. plantarum (Group III) showing white colonies on MRS agar plates on day 7.
Analysis of seminal parameters: On day 7, all animals from each Group were sacrificed and dissected for evaluation of seminal parameters viz. sperm count, motility and viability. The results showed that Group I, administered with PBS and Group III administered with L. plantarum, showed normal seminal parameters (sperm count, motility and viability) in both the sides in comparison to Group II (E. coli) where an alteration in seminal parameters was observed (Table 2).
Table 2: Seminal parameters of male mice inoculated with a single dose 104/108cfu of E. coli/ L. plantarum on day 7.

[Values represent Mean±SD]
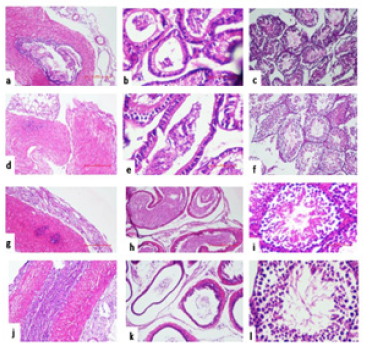
Histopathological Examination: The reproductive organs viz. testis, cauda and vas deferens obtained from mice were examined for histopathological changes on day 7. The Group I instilled with PBS and Subgroup L1 (104), and L2 (108) of Group III (L. plantarum) revealed normal tissue histology in all the reproductive organs (Figure 5). However, in Subgroup E1 (104) of Group II, the right testis showed mild hypo spermatogenesis and cauda demonstrated reduced number of mature spermatozoa. The left set of all the reproductive organs showed normal histology in both the Subgroups. In case of Subgroup E2 (108), the right set of organs viz. testis showed severe hypo spermatogenesis, cauda revealed lack of mature spermatozoa and vas deferens showed mild inflammation. The left side showed normal histology in all the organs (Figure 6).
Figure 5:Representative photomicrograph of histopathological examination of various reproductive organs viz. vas deferens, cauda and testis of mice administered in right vas deferens with single dose of i) PBS (Group I) (a-f) {Left side (a,b,c), Right side (d,e,f)}; ii) 104/108cfu/20μl of L. plantarum (g-l) {Left side (g,h,i), Right side (j,k,l)}on day 7.
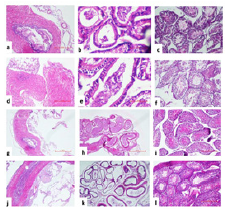
Figure 6:Representative photomicrograph of histopathological examination of various reproductive organs viz. vas deferens, cauda and testis of mice administered in right vas deferens with single dose of i) PBS (Group I) (a-f) {Left side (a,b,c), Right side (d,e,f)}; ii) 104/108cfu/20μl of E. coli (g-l) {Left side (g,h,i), Right side (j,k,l)}on day 7.
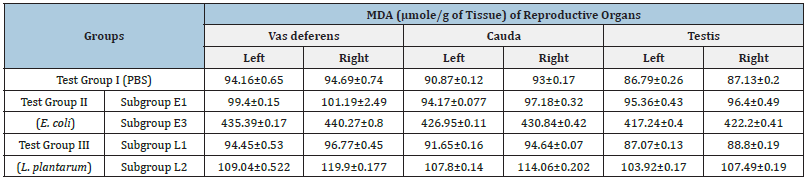
MDA estimation: To study the tissue damage caused by microorganisms, the levels of chief reactive aldehyde i.e., malondialdehyde (MDA) was estimated in reproductive organs, which are formed due to peroxidation of bio-membranes. Hence, reproductive organs viz. vas deferens, cauda and testis were studied for MDA (μmol/g of tissue) production. When the effect of different doses of E. coli (Group II) on MDA levels of all the reproductive organs was assessed, highly significant increase in MDA values were observed as compared to Group I (PBS) however, in case of Group III (L. plantarum) no significant changes in MDA values were observed as compared to Group I (Table 3).
Table 3: Effect of intravasal inoculation of different doses of E. coli/L. plantarum on MDA levels in reproductive organs on day 7.
[Values represent Mean±SD]
Combined effect of intra vas deferens inoculation of E. coli and L. plantarum on reproductive potential of male mice
Tissue somatic indices (TSI %): TSI (%) of the reproductive organs excised from groups of mice was determined on day 7. In Test Group I (PBS), the TSI (%) levels of reproductive organs of right side viz. vas deferens, cauda and testis were estimated to be 0.023±0.0025, 0.051±0.003 and 0.507±0.007 and the corresponding values in left side were 0.06±0.0026, 0.039±0.002 and 0.522±0.0015, respectively.
In Subgroup I of Test Group II, the TSI (%) of reproductive organs on right side were 0.025±0.001, 0.048±0.002 and 0.506±0.002 whereas on left side, the corresponding values were 0.061±0.0025, 0.038±0.002 and 0.521±0.002, respectively.
In subgroup II of Test Group II, the TSI (%) of reproductive organs on right side were 0.035±0.002, 0.033±0.002 and 0.38±0.002 whereas on left, the corresponding values were 0.033±0.002, 0.027±0.003 and 0.319±0.001, respectively.
Determination of bacterial load: The homogenates of all reproductive organs viz. vas deferens, cauda and testis of mice inoculated with E. coli (104/108cfu) and L. plantarum(108cfu), revealed log cfu of both E. coli and L. plantarum in all the 2 Subgroups of Test Group II whereas Group I (PBS) demonstrated no viable count (Table 4).
Table 4: Enumeration of bacterial load (in terms of log10cfu/g of tissue) from vas deferens, cauda and testis of mice challenged with 104/108 of E. coli with different doses (108) of L. plantarum/20μl intravasally on day 7.
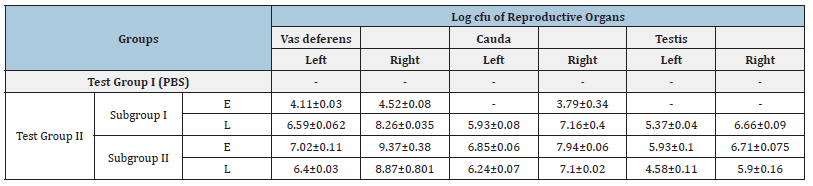
- No viable count, [Values represent Mean ± SD]
Analysis of seminal parameters: In order to evaluate the changes in seminal parameters viz. sperm count, motility and viability, all the mice were sacrificed on day 7 of Test Group I receiving PBS and Test Group II receiving single dose (104/108) of E. coli in combination with 108cfu of L. plantarum/20μl. Significant changes in seminal parameters of right side of Test Group II were observed as compared to left side. While Group I (PBS) showed normal seminal parameters (Table 5).
Table 5: Seminal parameters of male mice inoculated with PBS in Group I and of Test Group I, inoculated with the combination of 104/108 of E. coli with 108cfu of L. plantarum/20μl on day 7.
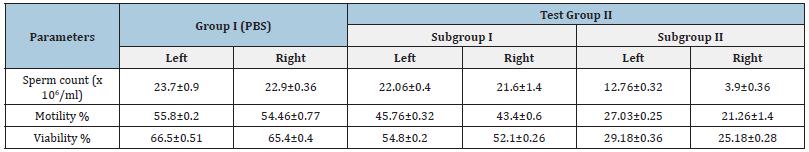
[Values represent Mean±SD]
Histopathological Examination: On day 7, the reproductive organs viz. vas deferens, cauda and testis obtained from mice inoculated with PBS (Test Group I) and of Test Group II inoculated with E. coli (104/108) and L. plantarum (108) in combinations were examined for histopathological changes. In Subgroup I the right and left set of all the reproductive organs showed normal histology (Figure 5). While in case of Subgroup II, the right set of organs viz. testis showed hypo spermatogenesis and vas deferens showed mild inflammation and the left side of organs revealed normal histology (Figure 6).
MDA estimation: Tissue damage caused by microorganisms can be studied by estimating the levels of MDA in reproductive organs viz. vas deferens, cauda and testis in terms of μmol/g of tissue. The MDA values of subgroup I of Test Group II showed insignificant changes in MDA levels as compared to Group I (PBS). However, the MDA values of subgroup II showed significant increase in MDA levels (Table 6).
Table 6: Effect of intravasal inoculation of PBS (Test Group I) and Test Group II with 104/108cfu of E. coli and 108cfu/20μl of L. plantarum on the levels of MDA on day 7.
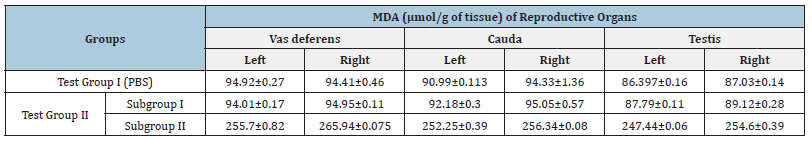
[Values represent Mean±SD]
Discussion
The exploitation of probiotics in the grassland of human reproduction is partial and mainly focuses on the female aspect. In this reverence, there are studies in which probiotics have been used as a therapy against bacterial vaginosis, submitting positive results in clinical trials [7]. Conversely, there are fewer accounts with respect to the effect of probiotics on male fertility in humans. Therefore, this experimental study was planned to investigate the ameliorative effect of L. plantarum over E. coli induced infertility in male mice. Although, many microorganisms have been greatly recognized to impede sperm parameters and thereby, reducing the fertilizing potential of males. But E. coli shows maximum potential among these microorganisms. Francesco et al. [6] had reported that E. coli is the second most common isolate from the infertile male. Thus, the clinical isolate of E. coli capable of causing 100% sperm agglutination was used in the present study. The standard strain of Lactobacillus plantarum MTCC 2621 was procured from the Institute of Microbial Technology, Sector 39, Chandigarh.
To study the relevance of the experiment, mice were administered intravasally with a single dose of 20μl of PBS and 104/108 of E. coli and L. plantarum as a single dose or in different combinations. Mice were sacrificed on day 7 and their impact on male reproductive potential was determined in terms of tissue somatic indices (%), bacterial load, seminal parameters, histopathological analysis and evaluation of reactive oxygen species in terms of MDA levels. The TSI (%) of reproductive organs (vas deferens, cauda and testis) was carried out to investigate their functional status ensuing the various experimental conditions. The results revealed alterations in TSI (%) values of reproductive organs, which lead to the morphological and functional abnormalities of these organs and this, could be attributed to urogenital tract infection by E. coli. However, no significant alterations in TSI (%) values of the various reproductive organs were observed in the groups of mice inoculated either with PBS or L. plantarum. These results are in harmony with the study carried out by Jantos et al. [7] wherein, they reported that intravasal administration of C. trachomatis biovar mouse pneumonitis induced enlargement of epididymides and caused significant alterations in relative organs weights. This highlights the fact that during pathological conditions, TSI becomes a predictor variable that has a dependence on body condition. Tissue somatic indices have been used as an indicator of Health Status and infectious diseases may alter Tissue somatic indices along with alterations in histopathological and hematological parameters.
In order to maintain an argument that changes in TSI (%) were only due to the pathogen under study, reisolation of both the microorganisms from reproductive tissues of mice was carried out. Bacterial enumeration studies showed bacterial load in both sets of reproductive organs of both microorganisms. In support of this, Hackett et al. [8] have also established that upon inoculation of E. coli into the left vas deferens of rabbits, and the organism could be recovered from both sets of reproductive organs. The colonization of E. coli (108) led to impairment of spermatogenesis as obvious by the absence of spermatozoa (azoospermia) being prominent in right vas deferens, whereas when 104cfu of E. coli was administered significant decrease in sperm count in right vas deferens was observed. The left vas deferens also revealed a reduction in spermiogram in both the subgroups. On the other hand, the PBS/ L. plantarum (104/108) administered groups revealed normal spermatozoa on the day of sacrifice. Diemier et al. [9] reported a substantial decrease in sperm concentration when inoculated intravasally with E. coli.
The result of colonization of these microorganisms on the histological architecture of reproductive tissue was also inspected. Hence, in the present study, mice in groups administered with (108) E. coli, the histopathological examination discovered severe changes in the reproductive organs in contrast to PBS/L. plantarum (104/108) receiving mice where no changes in the histological organization of organs were seen. Further, the right-side organs showed severe inflammation whereas; mild inflammation was seen in the organs of left side of E. coli (108) administered group. Similar results have been documented in earlier study that uropathogenic E. coli could result in hypospermatogenesis as an outcome of severe histopathological damage in terms of germ cell loss [10].
Reactive oxygen species are the important signaling molecules that play a major role in the sequence of inflammatory disorders. To evaluate severity of infection, tissue homogenates of both sides of reproductive organs of mice were assessed for lipid peroxidation, which was calculated in terms of levels of MDA. From the previous results, it was clear that (108) E. coli severely affects the right set of reproductive organs and therefore, the results indicated significantly high levels of MDA in the right set of reproductive organs of groups administered with E. coli in contrast to PBS/L. plantarum. These major rise in levels of MDA in E. coli group indicated increased oxidative stress in reproductive organs, which might have resulted in tissue injury, which further affects the process of spermatogenesis, thereby leading to the commencement of male infertility. In PBS/L. plantarum (104/108) administered group of mice, no significant changes in MDA levels were noticed. Masroor et al. [11] have correlated the seminal MDA levels in the patients of infertility wherein, they stated increased MDA levels in oligozoospermic patients in comparison to normozoospermic patients. From these results, it is clear that E. coli induced inflammation brings about endothelial dysfunction and tissue injury.
Since L. plantarum showed normal results, which were in comparison to PBS, studies were further carried out to determine the dose at which L. plantarum can ameliorate the devastating effects of E. coli. Therefore, the mice were administered with a single dose of 104cfu of E. coli in combination with 104sup>/108cfu of L. plantarum. When L. plantarum (108cfu>) was given in double the dose of E. coli (104), complete amelioration of all the parameters was observed such that the results were comparable to PBS group indicating that L. plantarum has suppressed the infection swayed by E. coli. Eaton et al. [12] obtained similar results, who found that L. reuteri suppresses the colonization and signs of disease due to E. coli in germ-free animals. He also reported that with an increase in the frequency of administration, protection against E. coli is enhanced. Abdel-Aziz et al. [13] found that oral administration of probiotic Saccharomyces cerevisiae enhances the growth performance and significantly mitigated the ochratoxin-induced toxicity by preventing oxidative stress and maintaining the glutathione content and protected against OTA-induced genotoxicity and spermatotoxicity. Yang et al. demonstrated the improved effect of L. acidophilus on the gastric inflammation caused by Helicobacter pylori.
When mice were administered with 108 of L. plantarum and 108 of E. coli in combination, the negative impact of E. coli dominated over L. plantarum. Thus, indicating that the ameliorative effect of L. plantarum is dependent on the dose being administered. Dosedependent results were also observed by Fang et al. [14] in which the effective dose of 108 cfu of L. rhamnosus 35 was able to cure rotaviral gastroenteritis in children when administered for 3 days.
Conclusion
It can be concluded from the present study that administration of E. coli alone can negatively affect the seminal parameters irrespective of dose by colonizing in the reproductive organs of mice, whereas administration of L. plantarum does not affect the same. In addition to this, double the dose of L. plantarum can ameliorate the negative impacts of E. coli when given in combination.
References
References
- FAO W (2001) Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Report of a joint FAO/WHO expert consultation on evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Cordoba, Argentina, pp. 1-4
- Reid G, Charbonneau D, Gonzalez S, Gardiner G, Erb J, et al. (2002) Ability of Lactobacillus GR-1 and RC-14 to stimulate host defences and reduce gut translocation and infectivity of Salmonella typhimurium. Journal of Food Science and Nutrition 7(2): 168-173.
- Hou D, Zhou X, Zhong X, Settles ML, Herring J, et al. (2013) Microbiota of the seminal fluid from healthy and infertile men. Fertil Steril 100(5): 1261-1269.
- Dardmeh F, Alipour H, Gazerani P, Vander HG, Brandsborg E, et al. (2017) Lactobacillus rhamnosus PB01 (DSM 14870) supplementation affects markers of sperm kinematic parameters in a diet-induced obesity mice model. PloS one 12(10): e0185964.
- Weng SL, Chiu CM, Lin FM, Huang WC, Liang C, et al. (2014) Bacterial communities in semen from men of infertile couples: metagenomic sequencing reveals relationships of seminal microbiota to semen quality. PloS one 9(10): 110152.
- Francesco MA, Negrini R, Ravizzola G, Galli P, Manca N (2011) Bacterial species present in the lower male genital tract: A five-year retrospective study. Eur J Contracept Reprod Health Care 16(1): 47-53.
- Jantos C, Baumgärtner W, Durchfeld B, Schiefer HG (1992) Experimental epididymitis due to Chlamydia trachomatis in rats. Infection and immunity 60(6): 2324-2328.
- Hackett RA, Huang TW, Berger RE (1988) Experimental Escherichia coli epididymitis in rabbits. Urology 32(3): 236-240.
- Diemer T, Huwe P, Ludwig M, Printzten SI, Michelmann HW, et al. (2003) Influence of autogenous leukocytes and Escherichia coli on sperm motility parameters in vitro. Andrologia 35: 100-105.
- Lu Y, Bhushan S, Tchatalbachev S, Marconi M, Bergmann M, et al. (2013) Necrosis is the dominant cell death pathway in uropathogenic Escherichia coli elicited epididymo-orchitis and is responsible for damage of rat testis. PLoS One 8(1): e52919.
- Masroor S, Muneshwar JN, Zingade S (2013) Estimation of seminal MDA levels in infertility patients. IOSR J Dent Med Sci 4(4): 2279-2861.
- Eaton KA, Honkala A, Auchtung TA, Britton RA (2011) Probiotic Lactobacillus reuteri ameliorates disease due to enterohemorrhagic Escherichia coli in germfree mice. Infection and immunity 79(1): 185-191.
- Abdel AKB, Farag IM, Tawfek NS, Nada SA, Amra HA, et al. (2010) Saccharomyces cereviciae ameliorates oxidative stress, genotoxicity and spermatotoxic effects induced by Ochratoxin A in male Albino Mice. New York Science Journal 3(11): 177-190.
- Fang SB, Lee HC, Hu JJ, Hou SY, Liu HL, et al. (2009) Dose-dependent effect of Lactobacillus rhamnosus on quantitative reduction of faecal rotavirus shedding in children. Journal of tropical pediatrics 55(5): 297-301.
© 2022 Vijay Prabha. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






