- Submissions

Full Text
Perceptions in Reproductive Medicine
A Comprehensive Review on Spinal Muscular Atrophy (SMA)
Sanjita Das* and Anupam Dubey
Noida Institute of Engineering and Technology (Pharmacy Institute), India
*Corresponding author: Sanjita Das, Noida Institute of Engineering and Technology (Pharmacy Institute), India
Submission: July 08, 2021;Published: July 23, 2021

ISSN: 2640-9666Volume4 Issue4
Abstract
Spinal Muscular Atrophy (SMA) is a genetic disease. Caused by deletion or mutation of SMN1, it is an autosomal recessive motor neuron disease. This disease is characterized by generalized muscle weakness and atrophy predominating in proximal limb muscles. Based on age of onset, it’s phenotype is classified into four grades of severity (SMA I, SMAII, SMAIII, SMA IV). This disease is caused by homozygous mutations of the motor neuron 1 (SMN1) gene, and the diagnostic test demonstrates in most patients the homozygous deletion of the SMN1 gene. Individuals at risk should be tested first and, in case of testing positive, the partner should be then analysed. The management of SMA should properly be followed-up coordination by an expert who is able to plan a multidisciplinary intervention that includes pulmonary, gastroenterology/nutrition, and orthopedic care is recommended. An effort has been made to focus on the diagnosis procedures followed by different effective management alternatives for SMA in the present review.
Keywords: Spinal Muscular Atrophy (SMA); Zolgensma; Genetic disorder; Gene therapy
Abbreviations: FDA: Food and Drug Administration; SMA: Spinal Muscular Atrophy; SMN: Survival Motor Neuron
Introduction
Characterized by degeneration of alpha motor neurons in the spinal cord, resulting in
progressive proximal muscle weakness and paralysis, Spinal Muscular Atrophy (SMA) is an
autosomal recessive neuromuscular disease. There is 4 subtypes of SMA exist which is identify
on the basis of clinical severities [1-5]. With an incidence estimated to be around 1 : 6,000 to
1 : 10,000 in newborns, SMA is one of the most frequent monogenic neurodegenerative diseases
[6-8]. It affects approximately 1 in 10,000 individuals and is the most common inherited
cause of childhood mortality, but this may soon change given recent developments [9]. The
U.S. Food and Drug Administration (FDA) approved Spinraza (nusinersen) for the treatment
of SMA on Dec. 23, 2016. The FDA approved Zolgensma the first gene-replacement therapy for
a neuromuscular disease in May 2019. With SMA with bi-allaetic mutations in the SMN1 gene,
Zolgensma is a one-time intravenous (into the vein) infusion for the treatment of pediatric
patients younger than 2 years of age with SMA including those who are presymptomatic at
diagnosis [10].
Zolgensma is the most effective and most expensive drug. Rs. 18 crores is the cost of per
dose. With resultant disuse and atrophy of voluntary muscles, Spinal Muscular Atrophy (SMA)
is an inherited neuromuscular disorder resulting in anterior horn cell degeneration [5]. SMA
is caused by a mutation in the Survival Motor Neuron (SMN1) gene. For proper function of
the motor neurons The SMN1 gene produces the SMN protein. The signals from the brain
and spinal cord to the muscles telling the muscles to move is send by the Motor neurons.
When motor neurons die and fail to send signals, the muscles waste away, or atrophy. Muscle
atrophy in SMA can lead to an inability to perform respiratory and motor functions properly
[11,12].
The patient shows different symptoms like
1. Areflexia, particularly in extremities
2. Overall muscle weakness, poor muscle tone, limpness or a
tendency to flop
3. Difficulty achieving developmental milestones, difficulty
sitting/standing/walking
4. In small children: adopting of a frog-leg position when
sitting (hips abducted and knees flexed)
5. Loss of strength of the respiratory muscles: weak cough,
weak cry (infants), accumulation of secretions in the lungs or
throat, respiratory distress
6. Bell-shaped torso (caused by using only abdominal
muscles for respiration) in severe SMA type
7. Fasciculations (twitching) of the tongue
8. Difficulty sucking or swallowing, poor feeding [10-14]
Classification
SMA can be classified into different categories depending on its onset and symptoms. The classification is as follows (Table 1 & Figure 1); [15-18]
Table 1: Classification of SMA on the basis of eponym and age of onset [16-19].

Figure 1: Clinical classification of SMA subtypes according to onset, milestones achieved, and clinical presentation. Typically associated SMN2 copy numbers are displayed [20].
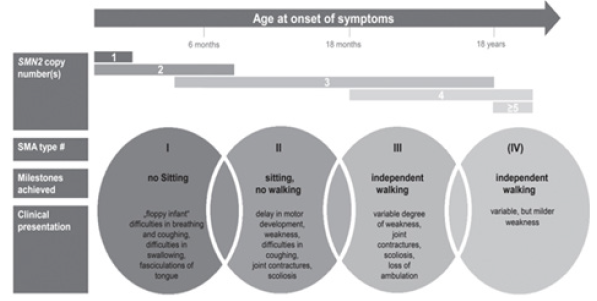
SMA 1: SMA type 1, or Werdnig-Hoffmann disease, is a serious
condition that usually appears before the age of 6 months. A child
may be born with breathing problems, which can be fatal within a
year without treatment [15-18].
SMA 2: Symptoms of SMA type 2 usually appear at the age of
6-18 months. The infant may learn to sit, but they will never be able
to stand or walk. In some cases, without treatment, the individual
may lose their ability to sit [15-18].
SMA 3: SMA type 3, or Kugelberg-Welander disease, appears
after the age of 18 months. The individual may have contractures, a
shortening of the muscles or tendons, which can prevent the joints
from moving freely [15-18].
SMA 4: It begins after the age of 21 years. The person will
have mild to moderate proximal weakness, which means that the
condition affects the muscles closest to the centre of the body [15-
18].
Etiology of SMA
People with SMA are either missing part of the SMN1 gene or have a changed (mutated) gene. SMN protein is produced by a healthy SMN1 gene. To survive and function properly motor genes need this protein. People with SMA don’t make enough SMN protein, and so the motor neurons shrink and die. As a result, voluntary movements can not be controlled by the brain, especially motion in the head, neck, arms and legs. On chromosome two almost identical SMN genes are present 5q13: the telomeric or SMN1 gene, which is the spinal muscular atrophy- determining gene, and the centromeric or SMN2 gene. By a single nucleotide the coding sequence of SMN2 differs from that of SMN1 (840C>T), which does not alter the aminoacidic sequence but results in alternative splicing of exon 7., SMN2 genes produce a reduced number of full-length transcripts (SMN-fl) and protein due to the alternative splicing of exon 7 , and a variable amount of mRNA lacking exon 7 (10% to 50%, SMN-del7) which give raise to a truncated and unstable protein [19-21]. Due to deletion or gene conversion of SMN1 to SMN2 about 95% of the patients have homozygous disruption of SMN1 [22]. About 3% of affected individuals are compound heterozygotes for deletion of one SMN1 allele and subtle intragenic mutations. All patients, however, retain at least one copy of SMN2, generally 2-While the severity of the loss of SMN1 is essential to the pathogenesis of SMA loss of SMN1 is essential to the pathogenesis of SMA. While type 3 and 4 generally have three or four, most SMA type I patients have two copies of SMN2, three SMN2 copies are common in SMA type II [23,24].
Diagnosis of SMA
For the diagnosis of SMA particularly in the severe variant of a
floppy baby or weak child clinical features are highly suggestive..
The intellect and attentiveness are always good. The weakness is
usually symmetrical and more proximal than distal; generally, it is
less in the arms than in the legs. By following methods the diagnosis
of SMA is mostly undertaken [25,26].
Blood test: The diagnosis of Spinal Muscular Atrophy is
performed by a genetic blood test.
EMG test: The electrical activity of a muscle or a group of
muscles is measured by Electromyography test.
Creatin kinase test: This test measures the high levels of
Creatin Kinase. This enzyme is released into the bloodstream by
deteriorating muscle.
Biopsy: Doctor removes small amount of muscle tissue and
send to it laboratory for examination in this test [26].
Treatment for SMA
Due to the resulting phenotypic spectrum of SMA it is generally
considered as a systemic disease [27]. The patients with SMA
requires the symptomatic management of respiratory, nutritional
and gastroenterological, orthopedic, and psychosocial issues [28].
Nonetheless, the implementation of standards of care is highly
variable and is influenced by cultural perspectives, socioeconomic
factors, and the availability of regional resources [29]. An updated
version of recommendations on diagnosing SMA and patient care
was published only recently due to advanced and improvements
in care over the last decade [30,31]. The FDA has approved three
medications to treat SMA: Nusinersen (Spinraza), onasemnogene
abeparvovec-xioi (Zolgensma) and risdiplam (Evrysdi) [32].
Antisense oligonucleotides Trusted Source (ASOs) are the drugs
where spinarza belongs which aim to target the underlying problem
by influencing the production of RNA. Genentech developed a
drug who is also a member of the Roche group, Evrysdi is another
effective agent for SMA which was developed in partnership with
SMA Foundation and PTC Therapeutics [32].
A one-time AAV-9-based gene transfer therapy which introduces
a full copy of the SMN1 gene is Onasemnogene abeparvovec. For the
treatment of Spinal Muscular Atrophy (SMA) in paediatric patients,
it is the most expensive medicine in the world. It was observed
after approval and reported an unprecedented survival rate at 24
months follow-up and unexpected acquisition of motor milestones
in 12 patients with infantile-onset spinal muscular atrophy
type 1, the most severe type of the disease [33]. After several
investigations, including approaches to increase muscle strength
and function by hyperacetylating agents such as valproic acid [34-
36] or phenylbutyrate [37], anabolic agents such as albuterol [38],
thyreotropin-releasing hormone [39] or growth-hormone [40] and
neuroprotective agents such as gabapentin [41,42], riluzol [43] and
olesoxime [44]. Actual therapeutic developments can be subdivided
into therapies aiming to modify the splicing of SMN2, replacing the
SMN1 gene, or upregulating muscle growth. Figure 2 summarizes
the therapeutic approaches discussed in the following sections and
illustrates the respective molecular mechanisms of action [20].
Figure 2: Illustration of therapeutic approaches in SMA involving molecular mechanisms of action [20] (modified illustration based on Farrar et al. [43] and Pechmann et al. [44]). FSTA = Fast Troponin Activator.

Conclusion
No disease modifying treatments are yet available despite our major progress to curb the infants’ death from the most common genetic disease of the spinal motor neutron. Several SMArestoring therapies are currently in the early phase clinic trials. The most effective treatment is costly and consequently research unaffordable. Gene therapy is allowing the clinical course to be substantially modified for the first time in the history of SMA. Additional therapeutic approaches are currently being taken at advanced stages of clinical development and are likely to expand the spectrum of drug treatment options for SMA. This will add to the complexity of care for patients with SMA. A timely diagnosis and treatment initiation are particularly important to achieve maximum treatment effects. To attain this goal, although it remains unclear when treatment should be initiated in patients presenting high numbers of SMN2 copies. With early onset SMA, the children show a higher rate of scoliosis during the first years of live despite the improved survival and motor developments of symptomatic patients. Greater awareness of this risk, and close monitoring of spinal deformities appear crucial to react early and enable the spine to be stabilized via medical orthoses. As many braces interfere with breathing in the more severely affected patients, choosing the ideal device can be difficult. Surgical interventions entailing ‘growing rod’ systems have been reported to be feasible in children with SMA1. Further experience in this field however is needed to balance the risks and benefits of these interventions. There are orthopedic devices for example standing frames – have not been used in most SMA type 1 patients, but they appear promising for the prophylaxis of joint contractures and to allow age-appropriate positioning even in more severely affected patients. This review may be a source to establish better management of SMA keeping in view the recent success of drug treatment in SMA, since many patients are left with a significant disease burden despite drug treatment.
References
- Mercuri E, Pera MC, Scoto M, Finkel R, Muntoni F (2020) Spinal muscular atrophy-insights and challenges in the treatment era. Nature Reviews Neurology 16(12): 706-715.
- Ross LF, Kwon JM (2019) Spinal muscular atrophy: Past, present, and future. Neo Reviews 20(8): e437-e451.
- Ogino S, Leonard DGB, Rennert H, Ewens WJ, Wilson RB (2002) Genetic risk assessment in carrier testing for spinal muscular atrophy. Am J Med Genet 110(4): 301-307.
- Lunn MR, Wang CH (2008) Spinal muscular atrophy. Lancet Lond Engl 371(9630): 2120-2133.
- Verhaart IEC, Robertson A, Wilson IJ, Rus AA, Cameron S, et al. (2017) Prevalence, incidence and carrier frequency of 5q-linked spinal muscular atrophy -a literature review. Orphanet J Rare Dis 12(1): 124.
- König K, Pechmann A, Thiele S, Walter MC, Schorling D, et al. (2019) De-duplicating patient records from three independent data sources reveals the incidence of rare neuromuscular disorders in Germany. Orphanet J Rare Dis 14: 152.
- Munsat TL (1991) International SMA Collaboration. Neuromuscul Disord 1: 81.
- Farrar MA, Vucic S, Johnston HM, Du Sart D, Kiernan MC (2013) Pathophysiological insights derived by natural history and motor function of spinal muscular atrophy. J Pediatr 162(1): 155-159.
- Zerres K, Schöneborn RS, Forrest E, Lusakowska A, Borkowska J, et al. (1997) A collaborative study on the natural history of childhood and juvenile onset proximal spinal muscular atrophy (type II and III SMA): 569 patients. J Neurol Sci 146(1): 67-72.
- Hahnen E, Forkert R, Marke C, Schöneborn RS, Schönling J, et al. (1995) Molecular analysis of candidate genes on chromosome 5q13 in autosomal recessive spinal muscular atrophy: Evidence of homozygous deletions of the SMN gene in unaffected individuals. Hum Mol Genet 4(10): 1927-1933.
- Peeters K, Chamova T, Jordanova A (2014) Clinical and genetic diversity of SMN1-negative proximal spinal muscular atrophies. Brain J Neurol 137(11): 2879-2896.
- Wirth B, Brichta L, Schrank B, Lochmüller H, Blick S, et al. (2006) Mildly affected patients with spinal muscular atrophy are partially protected by an increased SMN2 copy number. Hum Genet 119(4): 422-428.
- Calucho M, Bernal S, Alías L, March F, Venceslá A, et al. (2018) Correlation between SMA type and SMN2 copy number revisited: An analysis of 625 unrelated Spanish patients and a compilation of 2834 reported cases. Neuromuscul Disord NMD 28(3): 208-215.
- Lorson CL, Hahnen E, Androphy EJ, Wirth B (1999) A single nucleotide in the SMN gene regulates splicing and is responsible for spinal muscular atrophy. Proc Natl Acad Sci USA 96(11): 6307-6311.
- Zerres K, Schöneborn RS (1995) Natural history in proximal spinal muscular atrophy. Clinical analysis of 445 patients and suggestions for a modification of existing classifications. Arch Neurol 52(5): 518-523.
- Feldkötter M, Schwarzer V, Wirth R, Wienker TF, Wirth B (2002) Quantitative analyses of SMN1 and SMN2 based on real-time lightCycler PCR: Fast and highly reliable carrier testing and prediction of severity of spinal muscular atrophy. Am J Hum Genet 70(2): 358-368.
- Butchbach MER (2016) Copy Number Variations in the Survival Motor Neuron Genes: Implications for Spinal Muscular Atrophy and Other Neurodegenerative Diseases. Front Mol Biosci 3: 7.
- Prior TW, Swoboda KJ, Scott HD, Hejmanowski AQ (2004) Homozygous SMN1 deletions in unaffected family members and modification of the phenotype by SMN2. Am J Med Genet A 130A: 307-310.
- Schorling DC, Pechmanna A, Kirschner J (2020) Advances in Treatment of Spinal Muscular Atrophy - New Phenotypes, New Challenges, New Implications for Care. J Neuromuscul Dis 7(1): 1-13.
- Wirth B (2000) An update of the mutation spectrum of the Survival Motor Neuron gene (SMN1) in autosomal recessive spinal muscular atrophy (SMA). Hum Mut 15: 228-237.
- Gavrilov DK, Shi X, Das K, Gilliam TC, Wang CH (1998) Differential SMN2 expression associated with SMA severity. Nat Genet 20: 230-231.
- Schöneborn RS, Berg C, Zerres K, Betzler C, Grimm T, et al. (2009) Genotype-phenotype studies in infantile spinal muscular atrophy (SMA) type I in Germany: Implications for clinical trials and genetic counselling. Clin Genet 76: 168-178.
- Amico DA, Mercuri E, Tiziano FD, Bertini E (2011) Spinal muscular atrophy. Orphanet Journal of Rare Diseases 6: 71.
- Shroff A (2020) Spinal Muscular Atrophy. WebMD 1-6.
- Lipnick SL, Agniel DM, Aggarwal R, Makhortova NR, Finlayson SG, et al. (2019) Systemic nature of spinal muscular atrophy revealed by studying insurance claims. PloS One 14(3): e0213680.
- Wang CH, Finkel RS, Bertini ES, Schroth M, Simonds A, et al. (2007) Consensus statement for standard of care in spinal muscular atrophy. J Child Neurol 22(8): 1027-1049.
- Bladen CL, Thompson R, Jackson JM, Garland C, Wegel C, et al. (2014) Mapping the differences in care for 5,000 spinal muscular atrophy patients, a survey of 24 national registries in North America, Australasia and Europe. J Neurol 261: 152-163.
- Mercuri E, Finkel RS, Muntoni F, Wirth B, Montes J, et al. (2018) Diagnosis and management of spinal muscular atrophy: Part 1: Recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscul Disord NMD 28(2): 103-115.
- Finkel RS, Mercuri E, Meyer OH, Simonds AK, Schroth MK, et al. (2018) Diagnosis and management of spinal muscular atrophy: Part 2: Pulmonary and acute caremedications, supplements and immunizations; other organ systems; and ethics. Neuromuscul Disord NMD 28(3): 197-207.
- Krosschell KJ, Kissel JT, Townsend EL, Simeone SD, Zhang RZ, et al. (2018) Clinical trial of L-Carnitine and valproic acid in spinal muscular atrophy type I. Muscle Nerve 57(2): 193-199.
- Kissel JT, Elsheikh B, King WM, Freimer M, Scott CB, et al. (2014) SMA valiant trial: A prospective, double-blind, placebo-controlled trial of valproic acid in ambulatory adults with spinal muscular atrophy. Muscle Nerve 49(2): 187-192.
- Kissel JT, Scott CB, Reyna SP, Crawford TO, Simard LR, et al. (2011) SMA carnival trial part II: A prospective, single-armed trial of L-carnitine and valproic acid in ambulatory children with spinal muscular atrophy. PloS One 6(7): e21296.
- Mercuri E, Bertini E, Messina S, Solari A, Amico DA, et al. (2007) Randomized, double-blind, placebo-controlled trial of phenylbutyrate in spinal muscular atrophy. Neurology 68(1): 51-55.
- Kinali M, Mercuri E, Main M, De Biasia F, Karatza A, et al. (2002) Pilot trial of albuterol in spinal muscular atrophy. Neurology 59(4): 609-610.
- Tzeng AC, Cheng J, Fryczynski H, Niranjan V, Stitik T, et al. (2000) A study of thyrotropin-releasing hormone for the treatment of spinal muscular atrophy: A preliminary report. Am J Phys Med Rehabil 79(5): 435-440.
- Kirschner J, Schorling D, Hauschke D, Zimmermann RC, Wein U, et al. (2014) Somatropin treatment of spinal muscular atrophy: A placebo-controlled, double-blind crossover pilot study. Neuromuscul Disord NMD 24(2): 134-142.
- Merlini L, Solari A, Vita G, Bertini E, Minetti C, et al. (2003) Role of gabapentin in spinal muscular atrophy: Results of a multicenter, randomized Italian study. J Child Neurol 18(8): 537-541.
- Miller RG, Moore DH, Dronsky V, Bradley W, Barohn R, et al. (2001) A placebo-controlled trial of gabapentin in spinal muscular atrophy. J Neurol Sci 191(1-2): 127-131.
- Russman BS, Iannaccone ST, Samaha FJ (2003) A phase 1 trial of riluzole in spinal muscular atrophy. Arch Neurol 60(11): 1601-1603.
- Bertini E, Dessaud E, Mercuri E, Muntoni F, Kirschner J, et al. (2017) Safety and efficacy of olesoxime in patients with type 2 or non-ambulatory type 3 spinal muscular atrophy: A randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol 16(7): 513-522.
- Krosschell KJ, Kissel JT, Townsend EL, Simeone SD, Zhang RZ, et al. (2018) Clinical trial of L-Carnitine and valproic acid in spinal muscular atrophy type I. Muscle Nerve 57(2): 193-199.
- Goemans N (2017) Gene therapy for spinal muscular atrophy: Hope and caution. Lancet Neurol 20(4): 251-252.
- Farrar MA, Park SB, Vucic S, Carey KA, Turner BJ, et al. (2017) Emerging therapies and challenges in spinal muscular atrophy. Ann Neurol 81(13): 355-368.
- Pechmann A, Kirschner J (2017) Diagnosis and new treatment avenues in spinal muscular atrophy. Neuropediatrics 48(4): 273-281.
© 2021 Sanjita Das. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






