- Submissions

Full Text
Perceptions in Reproductive Medicine
Assessment of Endometrial Receptivity Assay (ERA) Test as a Diagnostic Tool and its Outcome in Patients with Previous IVF Failure
Saravanan L, Mahalakshmi S, Smitha M* and Sharma Nidhi
ARC International Fertility Center, India
*Corresponding author: Smitha M, ARC International Fertility Center, India
Submission: December 11, 2019;Published: November 16, 2019

ISSN: 2640-9666Volume3 Issue4
Abstract
Human implantation is a complex process requiring synchrony between a healthy embryo and a functionally competent or receptive endometrium. Diagnosis of endometrial receptivity (ER) has posed a challenge and so far, the most available tests have been subjective and lack accuracy and a predictive value. Microarray technology has allowed identification of the transcriptomic signature of the receptivity window of implantation (WOI). This technology has led to the development of a molecular diagnostic tool, the ER array (ERA) for diagnosis of ER. Use of this test in patients with recurrent implantation failure (RIF) has shown that the WOI is displaced in a most of these patients and the use of a personalized embryo transfer (pET) on the day designated by ERA improves reproductive performance. In this retrospective study, 74 patients with history of recurrent implantation failures who underwent Endometrial Receptivity Assay in ARC fertility centre, Saveetha medical college over a period of one year were analysed and 44 patients were receptive and 30 non receptive. Out of those 44 receptive patients 37(84.1%) were positive for clinical pregnancy and 7(23.3%) patients were negative for clinical pregnancy after performing pET. Among 30 of the non-receptive patients 7(15.9%) were found positive for clinical pregnancy and 23(76.7%) were found negative for clinical pregnancy. The p value was <0.001 which is statistically significant. Hence this study proves that doing ERA and performing Personal of clinical pregnancy rate and its outcome. Though larger studies are required to validate these results ERA has become a useful tool in our diagnostic armamentarium for ER.
Keywords: Endometrial receptivity; ERA; In-vitro fertilization; Recurrent implantation failure
Abbreviations: ER: Endometrial Receptivity; WOI: Window of Implantation; RIF: Recurrent Implantation Failure; PE: Phenotypes of Proliferative; ESE: Early Secretory; MSE: Mid‑Secretory; LSE: Late‑Secretory
Introduction
DNA microarray technology allows measurement of thousands of genes simultaneously, and its use to study the level of gene transcription in tissues has revolutionized medicine. The transcriptome reflects genes that are actively expressed at any given time within a specific cell population or tissue. Transcriptomics allows characterization of gene expression at the mRNA level, giving rise to a “sample‑specific” molecular profile or its “Transcriptomic Signature.” This permits characterization of tissue function or disease phenotype [1]. The transcriptome of the endometrium has been defined in all phases of the menstrual cycle [2,3] clustering of genes into four groups was identified and these were consistent with histological phenotypes of proliferative (PE), early secretory (ESE), mid‑secretory (MSE), and late‑secretory (LSE) phases. Extensive research on endometrial transcriptomics has allowed a provisional definition of the genomic signature of human ER and an understanding of alterations in the endometrium and ER that are associated with gynecological pathology and infertility. At a molecular level, the prereceptive/early secretory phase is characterized by increased metabolic activity in preparation for implantation. This leads to a predominance of products related to cell metabolism (fatty acids, lipids, eicosanoids, and amino alcohols), transport, and germ cell migration [4]. There is an inhibition of mitosis during this phase as suggested by the downregulation of several growth factors [3]. The receptive phase witnesses a “transcriptional awakening” or upregulation of most gene expression. Apart from a high level of metabolic and secretory activity there is also an upregulation of genes involved in the activation of the immune response [3]. During the late‑secretory phase, the WOI closes and in this phase, genes related to the immune response‑both cellular and humoral, blood coagulation, steroid bio‑synthesis, and prostaglandin metabolism are regulated [5].
Search for an adequate marker of ER, led to the development of a molecular diagnostic test the endometrial receptivity array (ERA). ERA consists of a customized microarray based on the transcriptomic signature of human ER, specifically when the human endometrium is receptive to blastocyst adhesion [6]. It has been designed to identify ER by comparing the genetic profile of a test sample with those of a luteinizing hormone (LH)+7 controls in a natural cycle, or on day 5 of P administration (P+5) after E2 priming in an HRT cycle. The test contains 238 genes that are differentially expressed between these profiles. This array is coupled to a computational predictor that can diagnose the personalized endometrial WOI of a given patient regardless of their endometrial histology. The gene signature used by the predictor was obtained by selecting those genes whose expression was consistent among three different models of ER: The natural cycle as the optimal model, the COH cycle as suboptimal, and the refractory endometrium as a negative control [6,7].
The bioinformatic predictor classifies an endometrial sample as “receptive” or “nonreceptive.” The “nonreceptive” ERA is further classified as perceptivity or post receptive giving an exact status of the endometrium at the time of biopsy [8]. Accuracy and consistency are the hallmarks of a good diagnostic test. ERA has a sensitivity and specificity of 0.99758 and 0.8857, respectively [6]. It has also been documented to have a high reproducibility. Gómez et al. [8] in their study demonstrated that the transcriptomic profile of the mid‑secretory phase endometrium did not change significantly between cycles or over relatively long periods (3 years). The study of the transcriptome of ER has brought to light the fact that the WOI is not fixed, as was believed earlier. Embryo‑endometrial synchrony is fundamental to successful implantation. The transcriptomic signature of the WOI can be used to define an individual’s personalized receptive window for use in IVF. It could also help in understanding the effect of different infertility treatments on the endometrial WOI and possibly identify the cause of treatment failure. Identifying ER changes in unexplained infertility, endometriosis, and other causes of infertility would help in providing more efficient treatment.
Aim of the Study
1. To identify the contribution of the endometrial factor in recurrent implantation failure.
2. To see if Pregnancy rates improved after using the personalized Window of implantation.
Type of study
A retrospective analysis of data done for a period from June 2018 to May 2019 investigated at the ARC Fertility centre, Saveetha Medical College and Hospital.
Material and Methods
This retrospective study examined 74 infertile women who underwent ER array (ERA) from June 2018 to May 2019.
Inclusion criteria
- Age 21-35.
- Patients with recurrent implantation failures (3 or more than 3 IVF failures).
- Patients having a normal ovarian reserve (follicle stimulating hormone <8, antral follicle count>10, and anti‑mullerian hormone (1.5 to 4ng/ml) were included.
- Good quality high grade embryos are preferred for embryo transfer.
Exclusion criteria
- Age >36.
- Low Ovarian Reserve.
- Patients with uncorrected uterine and adnexal pathology, e.g., hydrosalpinx, submucous polyps or myomas & previous difficult ET’s are excluded from the study.
Procedure
Endometrial biopsies were collected from the uterine cavity with the use of Pipelle catheters from Gynetics on day Progestrone+5 in an HRT cycle. The day of the EB in HRT cycle is after five full days of Progesterone impregnation, that is in the morning of the 6th day. After the biopsy, the endometrial tissue was transferred to a cryotube containing 1.5mL RNA stabilizing agent (Qiagen), vigorously shaken for a few seconds, and kept at 4 °C in refrigerator for 4h. Care was taken that the tissue was adequate and well‑immersed in the fluid provided. If the tissue is too much, there is RNA degradation, and if too little, sufficient RNA is not available for extraction. The samples were then transported at room temperature to the laboratory. The test results were available in 2 weeks. ERA test diagnosed the endometrium to be receptive (R) or nonreceptive (NR). Nonreceptive endometrium was further classified as pre or post receptive. A second ERA sample was taken for these patients on the suggested day, which means for prereceptive patients, a biopsy is taken one day behind in the subsequent cycle(Progestrone+6) and in post receptive patients in the next HRT cycle one day before ,the sample is taken (Progestrone+4). Patients with a receptive endometrium (Progestrone+5) underwent Frozen Embryo Transfer in a subsequent HRT cycle simulating the ERA cycle. In patients with a changed implantation window, FET was done based on the personalized WOI identified by ERA (pET). Two good quality blastocysts were transferred.
Result and Analysis
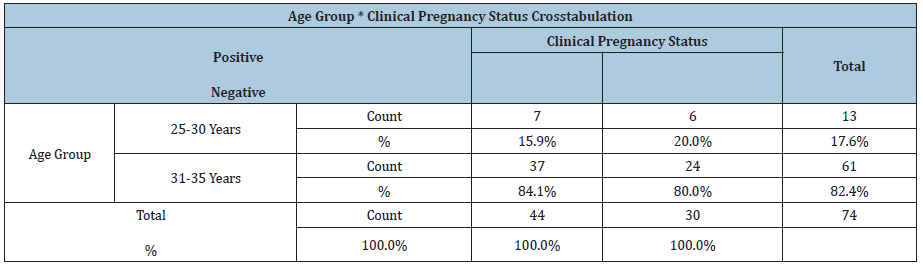
A total of 74 patients with history of recurrent implantation failures who underwent Endometrial Receptivity Assay in ARC fertility centre, Saveetha medical college over a period of one year were analysed in this study. The results were analysed used pearson chi square test. Out of 74 patients 13 were in the age group of 25- 30 years in which 7 patients turned positive for clinical pregnancy and the rest were in the age group of 31-35 years in which 37 patients turned positive for clinical pregnancy after performing ERA and personalized embryo transfer. Good quality high grade embryos are only considered for Personalised embryo transfer. The analysis depicts that if embryos grade was higher than the clinical pregnancy was most probably positive, and the embryo was found to be significant p=0.043. Out of 74 patients 44 patients (59.5%) turned to have an ERA report as receptive and the remaining 30(40.5%) of them had non receptive ERA report. In that 44 receptive patients, 37(84.1%) were positive for clinical pregnancy and 7(23.3%) patients were negative for clinical pregnancy after performing pET. Among 30 non receptive patients, 7(15.9%) were found positive for clinical pregnancy and 23(76.7%) were found negative for clinical pregnancy. The p value was <0.001 which is statistically significant. Hence this study proves that doing ERA and performing Personalised embryo transfer in recurrent IVF failure patients has a positive outcome and improves the success of clinical pregnancy rate and its outcome (Tables 1 & 2).
Table 1: Pearson chi-square=27.317** P< 0.001
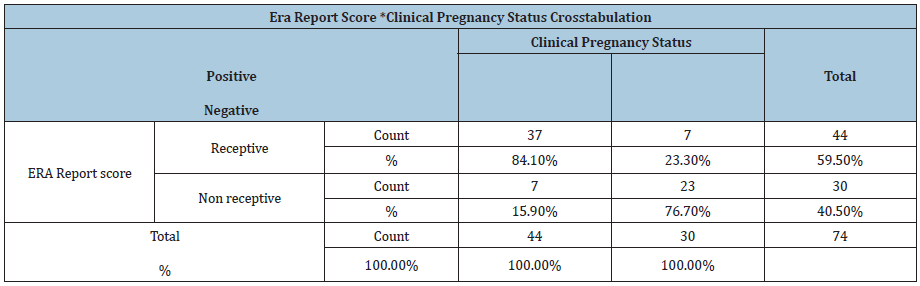
Table 2:
Discussion
The introduction of microarray technology has enabled rapid progress in the understanding of many biological functions and disease processes computing Omics with bioinformatic predictors has improved the diagnosis and subsequent treatment in diseases such as cancer [9]. Success in this area coupled with the identification of the transcriptomics of the receptive endometrium during natural and stimulated cycles [10], led to the development of a molecular diagnostic test to identify the WOI-ERA [6]. In the era of personalized medicine, a “one size fits all” policy is no longer acceptable. In IVF individualized ovarian stimulation, protocols are being promoted to optimize treatment. So far, for lack of an objective and accurate test, ER remains in a gray area. ERA is a step forward in improving IVF results through identification of the WOI and personalizing embryo transfer. The test has been shown to be accurate and reproducible and does not have the limitation of inter cycle variability. The clinical application of the test has been applied only in some clinics, and hence larger studies are required to validate it. In our study, we found that 40.5% women with RIF showed a displaced WOI. The paper by Ruiz-Alonso et al. [11] suggested an increased percentage of NR endometrium in the RIF group, but this did not reach statistical significance similar to our study. The PR, OPR, and IR in the RIF group improved after pET Out of 44 receptive patients, 37(84.1%) were positive for clinical pregnancy and 7(23.3%) patients were negative for clinical pregnancy after performing pET. Among 30 non receptive patients, 7(15.9%) were found positive for clinical pregnancy and 23(76.7%) were found negative for clinical pregnancy.
The p value was <0.001 which is statistically significant. Hence this study proves that doing ERA and performing Personalised embryo transfer in recurrent IVF failure patients has a positive outcome and improves the success of clinical pregnancy rate and its outcome. Though we saw an improvement of results, the numbers in our study are not high enough to draw definite conclusions. ERA is the only test available that can determine ER with accuracy. Since it is reproducible and does not change over a long period of time (1-2 years), it need not be repeated in the event of a delay in treatment. In the clinical setting, ERA definitely has a place in RIF where an endometrial factor could be the contributory cause in a quarter of the patients. In women with even 1 IVF-OD failure with the transfer of 2 good quality embryos, it is advisable to rule out an altered WOI. Defining a receptive window would avoid embryo wastage and emotional, physical, and financial distress. Its use in patients with adenomyosis, endometriosis, and chronic endometritis can prove beneficial, as these conditions are associated with an altered ER. Persistent thin or thick endometrium is also an indication for carrying out ERA. ERA is a valuable addition to our diagnostic armamentarium. The invasive nature of the test, the need for embryo vitrification and cost are some of its limitations. Much of the implantation process still remains to be unraveled. It has to be remembered that the embryo remains a major player in this equation and genetic testing of the embryo with an array of comparative genomic hybridization has shown improved Irs [12]. However, there are no reports suggesting a 100% success even after doing a pET with a euploid embryo. Material actors, especially the immune system involvement needs to be understood.
Conclusion
ERA is the most objective and accurate test available today for diagnosing ER. It has been used to define an altered WOI, and thus establish a personalized WOI for each patient. It has been shown to be a benefit in improving reproductive performance in patients with RIF. However, more studies are required to confirm these initial findings. It is limited by its invasive nature and associated costs.
References
- Nevins JR, Potti A (2007) Mining gene expression profiles: Expression signatures as cancer phenotypes. Nat Rev Genet 8(8): 601‑60
- Ponnampalam AP, Weston GC, Trajstman AC, Susil B, Rogers PA (2004) Molecular classification of human endometrial cycle stages by transcriptional profiling. Mol Hum Reprod 10(12): 879‑8
- Talbi S, Hamilton AE, Vo KC, Tulac S, Overgaard MT, et al. (2006) Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo‑ovulatory women. Endocrinology 147(3): 1097‑
- Gimeno DP, Alonso RM, Blesa D, Simón C (2014) Transcriptomics of the human endometrium. Int J Dev Biol 58(2-4): 127‑1
- Critchley HO, Kelly RW, Brenner RM, Baird DT (2001) The endocrinology of menstruation-a role for the immune system. Clin Endocrinol (Oxf) 55(6): 701‑7
- Gimeno DP, Horcajadas JA, Conejero MJA, Esteban FJ, Alamá P, et al. (2011) A genomic diagnostic tool for human endometrial receptivity based on the transcriptomic signature. Fertil Steril 95(1): 50-60.
- Horcajadas JA, Pellicer A, Simón C (2007) Wide genomic analysis of human endometrial receptivity: New times, new opportunities. Hum Reprod Update 13(1): 77‑
- Gómez GT, Alonso RM, Blesa D, Gimeno DP, Vilella F, et al. (2013) Profiling the gene signature of endometrial receptivity: Cinical results. Fertil Steril 99(4): 1078‑10
- Simon R (2003) Using DNA microarrays for diagnostic and prognostic prediction. Expert Rev Mol Diagn 3(5): 587-595.
- Liu Y, Lee KF, Ng EH, Yeung WS, Ho PC (2008) Gene expression profiling of human peri-implantation endometria between natural and stimulated cycles. Fertil Steril 90(6): 2152-2164.
- Alonso RM, Blesa D, Gimeno DP, Gómez E, Sánchez FM, et al. (2013) The endometrial receptivity array for diagnosis and personalized embryo transfer as a treatment for patients with repeated implantation failure. Fertil Steril 100(3): 818-824.
- Hellani A, Amero AK, Azouri J, El-Akoum S (2008) Successful pregnancies after application of array-comparative genomic hybridization in PGS-aneuploidy screening. Reprod Biomed Online 17(6): 841-847.
© 2019 Smitha M. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






