- Submissions

Full Text
Perceptions in Reproductive Medicine
Immuno-Interception of Human Chorionic Gonadotropin has Two Applications of Extraordinary Utility
Talwar GP* and Gupta Jagdish C
Talwar Research Foundation, E-8, Neb Valley, New Delhi-110068, India
*Corresponding author: Talwar GP, Talwar Research Foundation, New Delhi-110068, India
Submission: July 03, 2019;Published: July 09, 2019

ISSN: 2640-9666Volume3 Issue3
Opinion
We reported in 1994, the ability of a vaccine developed against hCG to prevent pregnancy in sexually active women of proven fertility [1]. Only one pregnancy took place in 1224 cycles. Eight women completed 30 cycles without becoming pregnant, nine were protected over 24-29 cycles, 12 for 18-23 cycles, 15 for 12-17 cycles and 21 for 6-11 cycles. All women continued to ovulate as was evident from luteal phase progesterone. They continued to have regular Menstrual cycles. This vaccine has now been converted to a genetically engineered vaccine so that it is amenable to industrial production. Figure 1 shows the design of the vaccine which consists of hCGβ linked to B chain of heat-labile enterotoxin of E. coli, as carrier [2]. The vaccine has been passed onto M/s Bharat Biotech, who will make the vaccine under GMP conditions. A new round of clinical trial will be carried out on the recombinant vaccine to prove its safety and efficacy. Approval has been received from the drugs controller general of India and institutional ethics committees to conduct these trials at the All India institute of medical sciences and Sir Gangaram hospital New Delhi. A new feature of this vaccine will be the use of both the DNA and protein form of the vaccine. Initial priming with 2 injections of the DNA form of the vaccine at zero and 15th day followed by the 3rd and 4th injections given on day 30 and 45 with the Protein form of the vaccine, raises considerably the quantum of antibody response.
Figure 1:Conceptualized structure of hCGβ-LTB vaccine. The carrier B chain of heat labile enterotoxin of E. coli (LTβ) is fused at C-terminal glutamine of hCGβ.

The previously reported vaccine heralded the encouraging possibility of birth control without any derangement of ovulation, hormonal profiles and menstrual regularity. Let us hope that the genetically engineered version of the same vaccine retains the same properties. Another highly interesting and useful application of the anti-hCG vaccine is expected for treatment of advanced stage invariably drugs-resistant cancers expressing ectopically hCG. HCG or its subunits have been reported to be expressed in a variety of nontrophoblastic cancers such as lung cancer [3], bladder carcinoma [4], colorectal carcinoma [5], pancreatic carcinoma [6], breast cancer [7], cervical carcinoma [8], oral cancers [9], vulva⁄vaginal cancers [10], prostate cancer [11] and gastric carcinomas [12]. The rationale behind the utility of the anti-hCG vaccine in treatment of hCG positive cancers lies in our observations that PiPP, a monoclonal antibody developed against hCG, kills such cancers. Given below are the observations on A549 lung cancer cell line. While FACS analysis showed that nearly 99% of the cells express hCG, incubation with different dilutions of monoclonal antibody PiPP in the presence of complement, ~96% of A549 cancer cells were killed (Figure 2). While many cancers expressing hCG are killed by the antibody directly in the presence of complement, some cancer cells, such as Molt4 T-lymphoblastic leukemia cells, though expressing hCG, are not killed by the antibody. However, if a conjugate of PiPP with curcumin is employed, it is effective in killing these cancer cells (Figure 3).
Figure 2:Dose dependent cytotoxicity exercised by the monoclonal anti-hCG-antibody PiPP on Lung Cancer cells (A549).
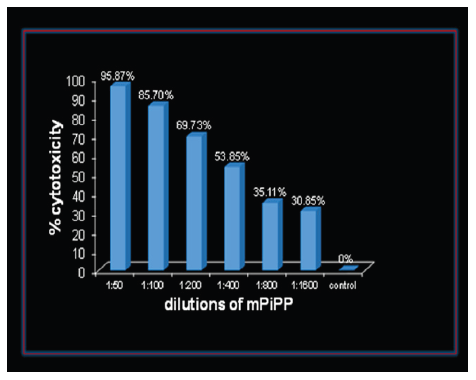
Figure 3:Photomicrograph of MOLT-4 cells after incubation with mouse monoclonal PiPP alone and PiPPcurcumin conjugate. Cells incubated in culture medium are used as control.

Acknowledgement
The clinical trial on the recombinant hCG vaccine is supported by a grant from the Indian council of Medical research.
References
© 2018 Talwar GP. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






