- Submissions

Full Text
Progress in Petrochemical Science
Process Modelling and Simulation of Reactive Distillation for the Synthesis of High Purity Mono Ethylene Glycol
Jofry Othman*, Norliza Abdul Rahman, Jarinah Mohd Ali and Siti Kartom Kamarudin
Chemical Engineering Programme, Faculty of Engineering and Built Environment, University Kebangsaan Malaysia, Malaysia
*Corresponding author:Jofry Othman, Chemical Engineering Programme, Faculty of Engineering and Built Environment, University Kebangsaan Malaysia, Malaysia
Submission: April 23, 2024;Published: May 17, 2024

ISSN 2637-8035Volume6 Issue3
Abstract
This study developed a suitable process modelling technique and simulation model for the non-catalytic synthesis of high purity Mono Ethylene Glycol (MEG) using the Reactive Distillation (RD) process. The feasibility of the RD process is demonstrated for performing the glycol reaction, dewatering and separation indistinguishable from the existing MEG production process with added advantages. Notably, a high purity MEG product of 99.8% is achieved within a single RD column configuration that avoids the need for the make-up of fresh demineralized water as in the current process. A working RD process model is generated that uses the established kinetics model parameters for MEG synthesis in combination with the equilibrium model and thermodynamic parameters in Aspen Plus® software. This included the power-law reaction variables for the main MEG synthesis along with the competing reactions that generate Diethylene Glycol (DEG), Triethylene Glycol (TEG) and Tetra Ethylene Glycol (TTEG) by-products. Hydrodynamics conditions for this process are also produced using a suitably packed column with the counter-current flow configuration of gas and liquid phases. The hydrodynamic variables including packing specification, maximum stage liquid holdup and maximum liquid superficial velocity, are generated to establish a maximum of 47.2% packing capacity and a low-pressure drop of 0.1kPa. Remarkably, compared with the conventional process RD promoted a 3.4% increase in MEG yield and a 53.2% reduction in energy usage. A lower water-to-ethylene oxide feed ratio of 12.9 is found to be suitable against the conventional ratio of 20 which reduces the separation loading while achieving a higher affinity for MEG selectivity of 91.4%. This subsequently generated a low energy usage through the utilization of reaction heat for separation with a net energy release of 0.24MW which lowers the operation cost.
Keywords:Reactive system; Mono ethylene glycol; Equilibrium model; Ethylene oxide
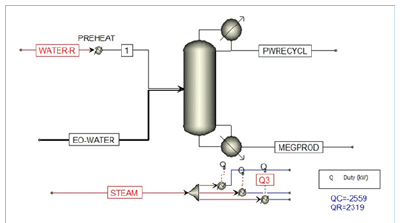
Figure 1:Reactive Distillation Column for MEG Production
Introduction
The flexibility and appealing properties of Mono Ethylene Glycol (MEG) allow its wide applications for PET (Polyethylene terephthalate) bottle resins, antifreeze, polyester fiber, polyester film, aircraft and runway deicing fluids [1,2]. At the present, the conventional MEG production process based upon EO hydration requires a high waterto-ethylene oxide feed ratio of 20:1 for achieving sensible MEG yield against competing reactions that increases energy requirement [3- 6]. This processing method can deliver a reasonable MEG yield at molar selectivity of MEG, Diethylene Glycol (DEG) and Triethylene Glycol (TEG) around 88-11-1 separately [3,4]. Further MEG yield increase is limited by constraints on the reaction temperature, pressure and residence time. This is further comprehended by using the conventional separation method that has low effective energy and thermodynamic efficiency. MEG production largely relies upon a non-catalytic EO hydration process with an exothermic reaction that increases the reaction rate at a higher temperature. This uses a lean EO reactant that is blended with high purity condensate to create a homogenous feed blend into the glycol reactor. Typically, the glycol reactor utilizes a tubular tube adiabatic reactor design to accomplish to achieve an appropriately controlled turbulent flow and prevention of back-mixing as shown in Figure 1. In particular, a high water-to- ethylene oxide proportion is required to accomplish sensible MEG yield and avoid EO vapour breakthrough that however results in high energy-intensive separation [3-6].
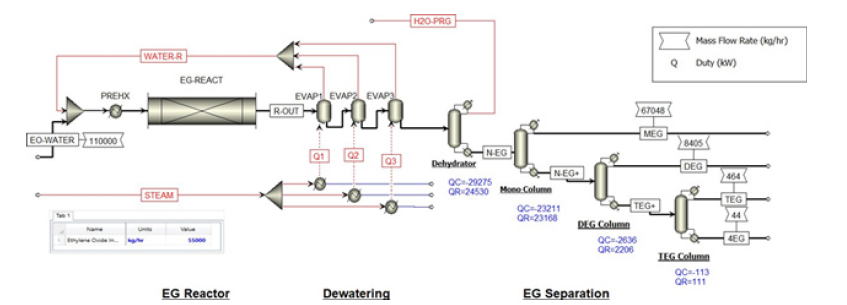
Figure 2:Process flow diagram of the current MEG production.
RD is an efficient process intensification approach that integrates chemical reaction and distillation in a single column to achieve higher process performance [7-9]. However, no appropriate RD model is presently available that can appropriately describe a combined MEG reaction, dewatering and separation functions to meet MEG product specifications. As an example, studies by Steffen V et al. [5], Athira GP et al. [10] and Lijing Z et al. [11] and proposed a preliminary RD concept for MEG production using an initial estimation point approach to obtain MEG purity of 94.5% during steady state. These however are unable to meet the high purity MEG product specification that is also not designed to handle disturbances in either feed flow or feed composition. In addition, studies on different modelling aspects of reactive distillation by Steffen V et al. [5] and Yingjie M et al. [6] have identified major design factors and the likely occurrence of multiple steady states that could result in the accumulation of volatile EO at the column overhead. Moreover, a lack of study on column physical constraints exists, for example, the presence of flooding or weeping along with the absence of control on EO breakthrough that may result in volatile EO accumulation at the top section of the column.
This study reports on the RD process that overcomes the present limitations in MEG production by using a lower a high water-to-ethylene oxide ratio that is achievable through integrating MEG reaction, dewatering and product separation. Description of an RD model that directly synthesizes lean aqueous EO and high purity water feedstocks in a single column to produce a high purity MEG to meet the product specification is included. Steadystate simulations using Aspen Plus® version 12 software were conducted on non- catalytic EO hydration for both conventional and RD processes. Surprisingly, the single RD column manages to produce a 99.8% purity MEG production using an aqueous EO feed at 32% w/w concentration and recycled process water containing 0.001-mole fraction glycol. In addition, the optimization challenges of the many entangled design variables within RD are overcome heuristically to generate the optimum operating pressure. This includes the analysis of the impact of liquid and vapour equilibria on individual stages and Gibbs free energy results that showed the lowest deviation in addition to the establishment of key processing parameters.
Previous Research and the Novelty of this Study
From literature progress, the previous studies on RD application for MEG production can be segregated into three (3) development phases. The first phase involves several early studies on RDC for MEG production that provided the initial background on process synthesis, design concept and Advanced Process Control (APC). As example, several papers by García A [12] and Aqar DY et al. [13] have proposed preliminary findings on the synthesis and RD design using a Mixed-Integer Nonlinear Programming (MINLP) concept. In addition, Blatkiewicz M et al. [14], Cho M et al. [15] and Dai SB et al. [16] have provided further investigations into RD control system development involving a combined temperature controller and feed-forward compensator approach, nonlinear inversionbased controller and observer-based input/output linearizing compensator. On another aspect, Fonseca JD et al. [17] suggested RD startup configuration and different operating strategies that consider multiple steady states for ethylene glycol production. A study by Li G et al. [18] has provided early concept design on RDC and control structure with improvement in key process parameters such as operation at essentially neat EO, stoichiometric balance between reactants and reasonable control of product purity. This design however requires operation at significantly higher pressure and temperature that require an effective control structure to control discrepancy between reaction and separation operating envelopes.
Next, the second phase of studies attempt to improve the theoretical understanding on RD process for MEG production. Specifically, these provide screening criteria on RD for MEG production, investigation on alternate processing route, improvement in control system and RD configuration. As example, Li X et al. [19] and Huang W et al. [20] have investigated the RD separation process using syngas feedstock, but these do not involve EO hydration to MEG. Another study by Jana AK [21] proposed a nonlinear control system for ethylene glycol RD involving Neuro Estimator (NE)-based Inferential Extended Generic Model Controller (IEGMC). A study on RD configuration by Ding L et al. [4] provided preliminary analysis on Side Reactor Concept (SRC) involving a non-reactive column combining with side reactors for EO hydration to EG. Additionally, Li X et al. [19] investigated a preliminary RD control concept for MEG production using two reactive distillation columns for accurately balancing EO and water reactants usage as reaction stoichiometry. Another RD approach suggests selection criteria of phase equilibrium, reaction equilibrium and integration between reaction and separation parameters [22,23]. This includes a study by Yamaki T et al. [24] that suggested two determining criteria on RD for MEG involving the residence time for reaction and acceptable MEG product purity exiting RD bottom. In technological perspective, RD for MEG can potentially reduce the DEG formation as it drives to maintain low EO concentration from controlling higher EO volatility and utilization of heat of reaction for vaporizing liquid mixtures in column. Therefore, the consideration on suitability of RD for MEG should include both preferred process criteria and possible constraints that form key process envelope. Specifically, Zhang Q et al. [25] has suggested that the key RD process constraints for MEG application should include maximum volatility, residence time, flow limitation and deviation from optimum process temperature and pressure needed for reaction and separation. These however are unable to meet the high purity MEG product specification and is not designed to handle disturbances in either feed flow or feed composition.
Correspondently, lack of fundamental understanding persists on the critical RD design factors, basis of conceptual design and optimum operating parameters for MEG production. The existing literature does not address such a simulation strategy for studying the transition behavior from the nonreactive to the equilibrium reactive limits. Uncertainties exist on the potential existence of restricted parameters that prevent wider optimization between favorable reaction in RD column and unfavorable distillation occurring in reboiler. This led into an inadequate nonlinear control strategy for RD process that can give good performance and stability, lack of proper mechanism that describes the nonlinear coupling of reaction, transport and phase equilibrium behavior, and conceptual RD design that provides improved performance than conventional MEG production. Furthermore, the integrated nature of RD process is also known to contribute into higher design variables and lower degree of freedom than conventional MEG process. In conclusion, no RD model is available that can appropriately describe a combined MEG reaction, dewatering and separation functions to meet high purity MEG product specifications. Therefore, this study developed RD process that can lead into an improved MEG production performance without causing inadvertent consequences such as process safety issues. The novelty of this process is that the parameters used for non- catalytic MEG synthesis are modified to identify non idealities and create an integral relationship between equilibrium models and rate-based models in a RD process. This enables an accurate determination of the physical properties of glycol mixture, within the target operating envelope and RD process that is based on a modified distillation process. This allows a higher packing factor to be incorporated into the model in order to improve its separation capacity, while at the same time providing an easier control scheme and further validation of the model based on test data.
Method
Estimation of modelling parameters
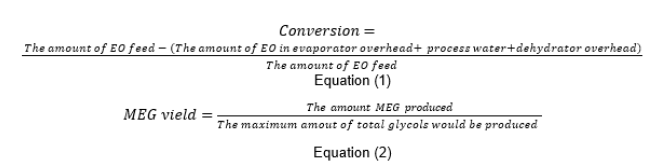
A Cubic-Plus-Association (CPA) equation of state is selected as it provides good suitability for glycols and water mixture including compositional phase diagrams for the key mixture components i.e. MEG-water, EO-water and MEG-EO. Table 1 shows the generated binary interaction parameters represented by ‘i’ and ‘j’ that are used to compute the non- ideality of these mixture components. In this way, it is feasible to provide an accurate prediction of the physical properties of the glycol reaction mixture within appropriate operating temperature and pressure. Modelling parameters at equilibrium conditions involving thermodynamic phase diagrams and compositional variables are generated using the modelling methodology in Figure 2 with an inside-out algorithm in RADFRAC. Reaction rate expressions from Akpa JG et al. [3] are used to describe the main MEG synthesis and competing reactions with suitable modelling assumptions i.e. RD column is in steady state, attain perfect mixing at constant vapor and liquid rates in all stages, and follow bulk reaction behavior with unequal temperatures in both liquid and vapour phases. By solving the column differential equations and other equations as in Figure 3, other parameters involving liquid phase temperature, mass and heat transfer rates, the extent of EO feed vaporization, column bottom composition and reboiler composition are determined.
Table 1:Binary interaction parameters for CPA EOS property.
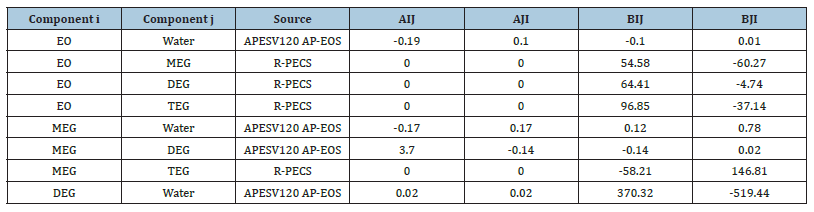
Figure 3:Steady-state reactive distillation modelling for MEG production.
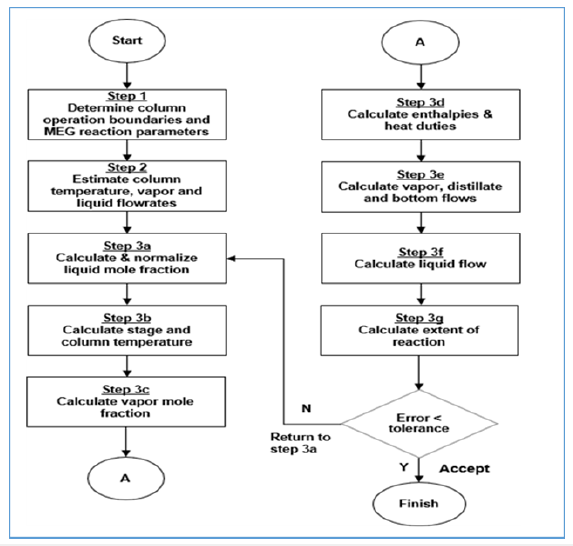
Figure 4:Simplified modelling equations.
Reaction synthesis procedure
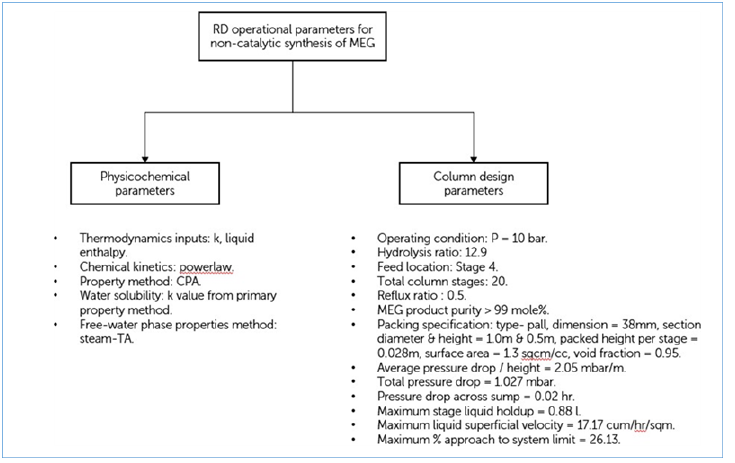
For generating the suitable RD operational parameters, several parameters are specified to form the fixed input for this study as shown in Figure 4. An aqueous EO feed at 32% w/w concentration and preheated recycled water containing 0.001-mole fraction glycol are mixed at a hydrolysis ratio of 12.9 to form a saturated liquid. This mixture is then subjected to a packed reactive distillation column with 20 stages, a low reflux ratio of 0.5 and 10 bar column pressure for the synthesis of MEG and evaporation of water. The remaining stages are then used for purifying MEG into a high purity product > 99 mole% and with the evaporated water is continuously recycled to the RD column feed preheater. Selected fixed parameters are used in the inside-out method as primary iteration variables that underwent stepwise integration for finalizing into stage model equations.
Figure 5:Key parameters of MEG RD column.
Results and Discussion
Equilibrium thermodynamic analysis
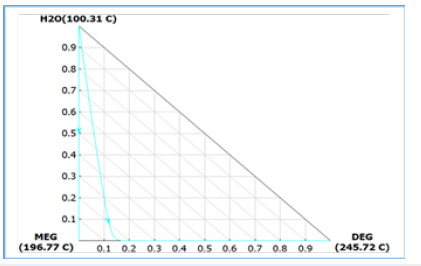
Figure 5 shows the generated ternary phase diagram for the key components that represent the phase behavior of reactive mixtures over the composition range. This alludes to the phase separation envelope and modelling parameters for MEG reaction and separation in both liquid and vapour phases. Combining with the binary interaction parameters it is possible to estimate the possible phase splitting that occurs during reactive distillation.
Figure 6:Tertiary diagram with phase envelope.
Kinetics study
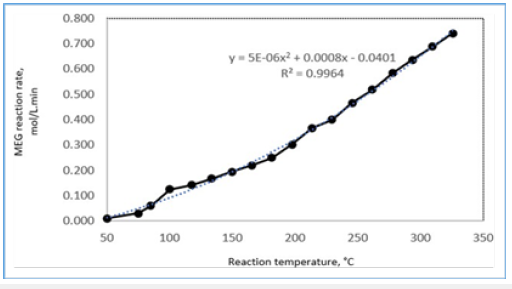
Split thermodynamic models were used in vapour and liquid phases to provide higher flexibility and better property estimation. The physical and thermal properties of the process liquid mixture were derived from the presence of glycol components (MEG, DEG, TEG) using the database parameters as given by Yaws, (2003). Similarly, vapour phase properties were generated by using the Wilson method for vapour thermal conductivity, vapour viscosity method and vapour thermal properties to represent the non-ideal behavior. These provided the ability to model complicated Vapour- Liquid Equilibrium (VLE) and Liquid-Liquid Equilibrium (LLE) behaviors in reactive distillation. The kinetic effects in MEG reactive system were determined, inter alia, by the steady-state profiles of temperature, vapour and liquid flow rates and liquid composition (Figure 6).
Figure 7:MEG reaction rate effects against reaction temperature.
Simulation of MEG synthesis process
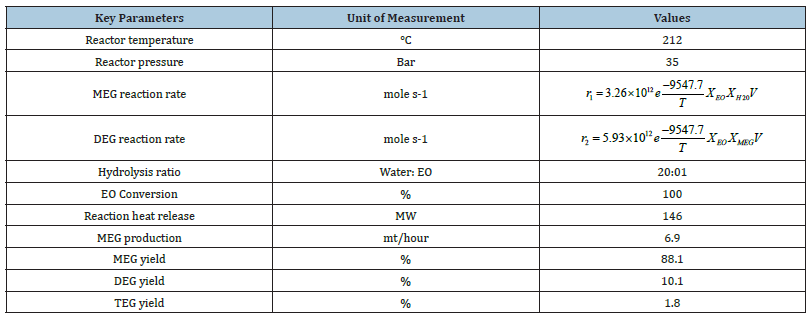
Simulation of the present MEG production: Steady-state simulations using Aspen Plus software v12 are generated on both the current MEG production and RD processes. Figure 1 portrays the conventional process scheme that consists of a plug flow reactor, multi-effect evaporators and vacuum distillation columns for performing MEG synthesis, dewatering and MEG purification. Aqueous EO and condensate feeds are heated up in preheater up to the operating reactor temperature of 170C at 35barg. Three multieffect evaporators provide separation of glycol reaction products and removal of reaction water for usage as process recycling water. Consequently, this simulation continued for producing 6.63mt/ hour of MEG production using an aqueous EO feed of 110mt/hour at 25% w/w concentration and high purity water of 547.6mt/hour. The outcomes demonstrate that a high hydrolysis ratio is needed to accomplish reasonable MEG selectivity that adds to high energy for removing excess reaction water and cost-intensive separation. The excess hydrolysis water reacting with EO produces a maximum MEG yield at molar selectivity of MEG, DEG and TEG of 88.1 - 10.1 - 1.8 respectively as shown in Table 2. These results are consistent with the previous finding by Akpa JG et al. [3], Steffen V et al. [5] and Yingjie M et al. [6] that excess water (almost 20 times more) is required to achieve high MEG selectivity and at least 90% conversion of EO to glycols. Besides, this simulation determined that additional adjustments in reaction parameters such as reaction temperature or pressure will not further increase the MEG yield.
Table 2:Simulation results of conventional MEG production.
Study of reactive distillation for MEG production: The defined kinetics model parameters and power-law reaction variables for MEG synthesis along with the competing by-product reactions are used to generate a new RD process model. Both EO and water reactants are consumed in the packed RD column exhibiting a rapid EO hydrolysis that results in a counter-current flow along the reactive stages. The recycled process water is introduced into stage 2 to mitigate the high EO vaporization towards the top of the column, provide sufficient hydrolysis waste for MEG reaction and allow adequate control on the overhead reflux drum. It was found that it is possible to maintain low EO and MEG concentrations in the reactive stages that promote a lower tendency for DEG coproduct generation and hence a minimal potential of further TEG formation. The MEG synthesis performance including process stream properties, conditions, composition and component split fraction are shown in Table 3 and Table 4. Reboiler is modelled as kettle type and condenser as total reboiler with their efficiencies at unity attaining vapour and liquid equilibrium. A low reflux ratio of 0.5 and boil-up ratio of 80 are used for the full simulation including regulating MEG bottom flow composition to attain a high product purity of 99.8%. The feed to this RD column avoids the need for fresh demineralized water use as in conventional process by using preheated recycled process water containing 0.001-mole fraction glycol that is combined with an aqueous EO at 32% w/w concentration. As displayed in Figure 7, this level loop control balances the reaction stoichiometry by adjusting the water feed flowrate in reaction with aqueous EO feed to match the target hydrolysis ratio of 12.9.
Table 3:Process streams of MEG synthesis using reactive distillation.
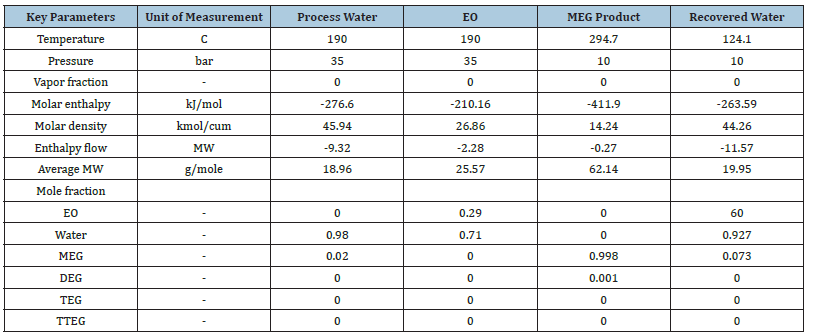
Table 4:Component split fraction.
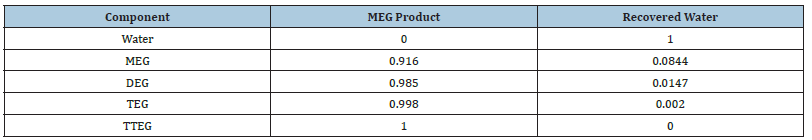
Figure 8:RD column configuration.
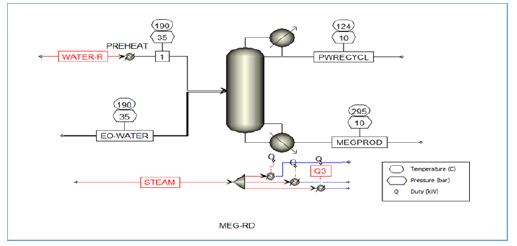
A strong dependency of MEG with temperature is shown in Figure 5 with an average of a double MEG reaction rate observed with a 50 °C increase in reaction temperature. Even though the initial reaction rate decreases with the initial temperature increment, this however stabilized upon activation showing a rapid reaction rate that is contributed by the high EO volatility and water solubility. MEG reaction and separation occur in stages 1 to 4 while the remaining stages are used as rectification to purify the MEG product towards 99.8-molewt %. Additionally, the volatility difference between glycols and EO water mixture favours the separation of glycols into column bottom with lighter feeds towards the upper section that allows most of the reaction to occur at the top section. Upon reaction progress, overhead distillate is being continuously removed and subsequently the vapour distillate rate of 160kmol/hr is determined as the optimum parameter to attain target MEG purity as shown in Figure 8. In addition, the EO feed location at stage 4 is found to provide both high purity MEG and a 53.2% reduction in energy usage as compared to the conventional process (Figure 9,10).
Figure 9:Effect of the vapour distillate rate on MEG bottom product purity.
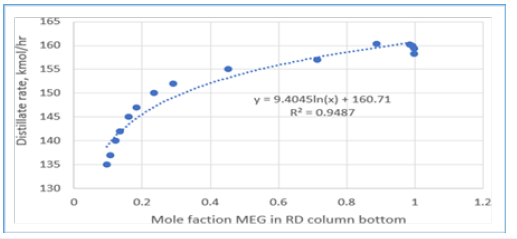
Figure 10:MEG purity effect from different EO feed location.
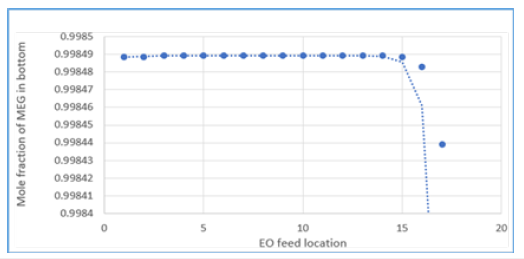
Figure 11:Energy usage effect from different EO feed location.
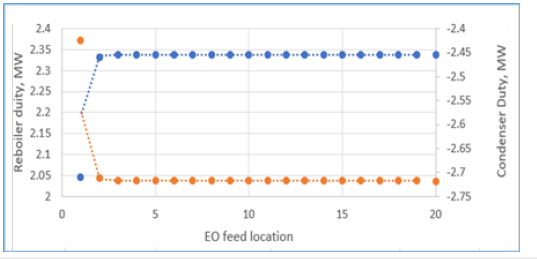
The entering EO feed reacts with excess water to form a low concentration of feed EO and MEG the reactive stages that result in water mole fraction close to unity. These EO and MEG concentrations promote a lower tendency of DEG generation that suppress the formation of undesired TEG by-product. The generated composition profiles of the key components have considered the effects of concentration, vapour flow and liquid flow along the column stages as displayed in Figure 11 & 12. A good correlation of the liquid and vapour flow profiles against column temperature is achieved from the vaporization of the liquid mixture from reaction heat. Moreover, the reaction heat transfer onto the process liquid changes the liquid and vapour flow along the reactive stages that also lower the required number of column stages to achieve high MEG purity. With the thermodynamic feasibility at a common operational envelope, a temperature range of 190-280 °C along with a 10-bar column pressure has shown good suitability for generating MEG synthesis with high MEG purity. Subsequently, the optimum RD column pressure that balances high reaction rate, stripping operation, reboiler ratio and optimization of liquid holdup volume is determined (Figures 13-15).
Figure 12:Composition profiles of the key components.
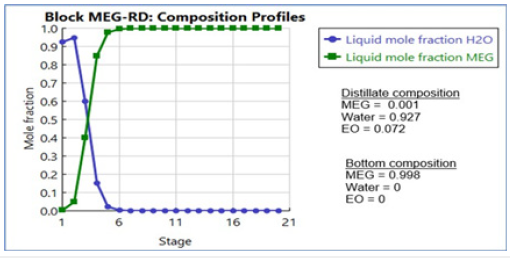
Figure 13:Liquid and vapor flow profiles in MEG RD column.
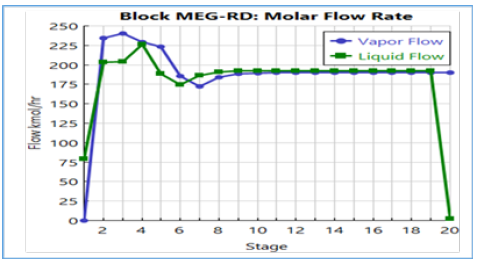
Figure 14:Temperature profile with active constraint on molar flow rate.
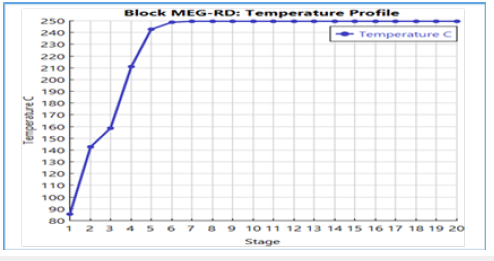
Figure 15:Vapor-liquid k-value profiles.
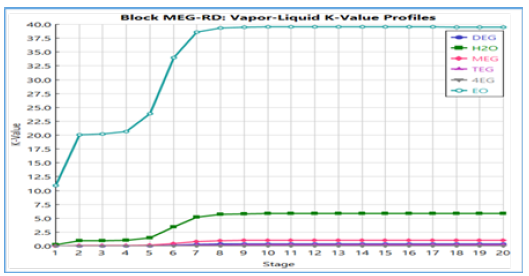
Figure 16:Column pressure effects to reboiler duty, MEG yield and purity.
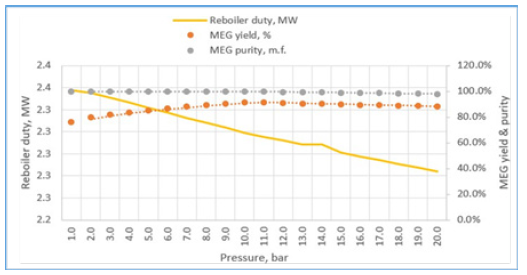
In addition, the hydrodynamics analysis is performed to create an optimized configuration of column internals, packing geometry and design parameters as shown in Table 5. Key variables that contribute to steady vapour and liquid flow distributions along the column stages are generated such as suitable packing specification, maximum stage liquid holdup and liquid superficial velocity. As result, an adequate column design capacity is attained with 48.1% packing capacity and 32.9% approach towards system limit with a low-pressure drop of 1.2mbar (Figures 16-19). It is shown that the RD process system can attain an efficient MEG reaction rate with a good separation performance to produce a 3.4% increase in MEG yield and a 53.2% reduction in energy usage as compared to the existing process. This is possible through the rapid removal of MEG as the most volatile product from the reaction system and exploiting the maximum driving force in reactive distillation with a reduced residence time. Additionally, the external steam requirement is significantly minimized with the use of a lower hydrolysis ratio and reaction heat utilization i.e. a net energy release of 0.24MW that lowers the operation cost. In parallel, an enhanced process safety performance is gained through continuous removal of the EO reaction products and simpler MEG synthesis control that improve operational reliability to lower the possibility of out-of- control reaction such as EO vapour breakthrough. This RD process model shows a dependency on related properties for MEG synthesis (such as kinetics, hold-ups) and separation features (such as thermodynamics and vapour-liquid equilibrium) that avoid undesired process constraints (Table 6). Finally, a comparison between the results from this work and previous studies by Ding L et al. [4] and Steffen V et al. [5] is provided in Table 7 on process performance parameters involving feed composition, system configuration and reaction water recyclability, MEG purity and yield. Comparable results are achieved on EO conversion, reactive column configuration and pressure (Table 8). Improvements are shown in terms of the lower composition of EO reactant, higher MEG purity that meets product specification and higher MEG yield [26].
Table 5:Hydraulic analysis of MEG RD.
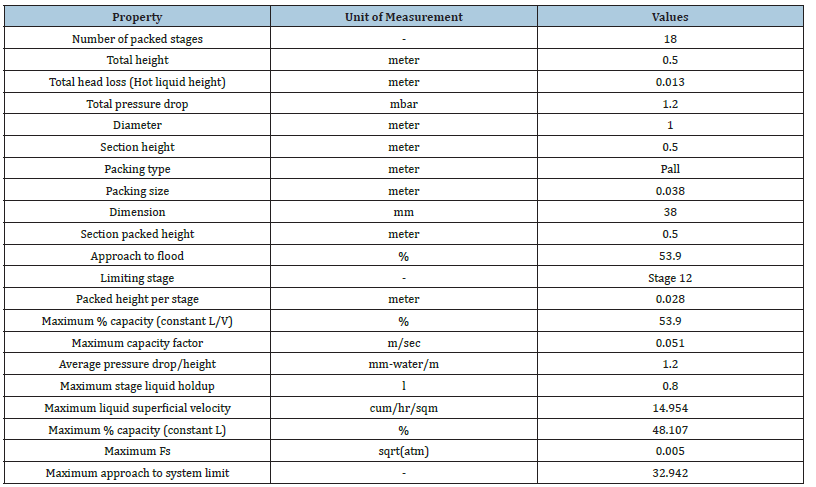
Figure 17:Hydraulic profile of the EO feed stage.
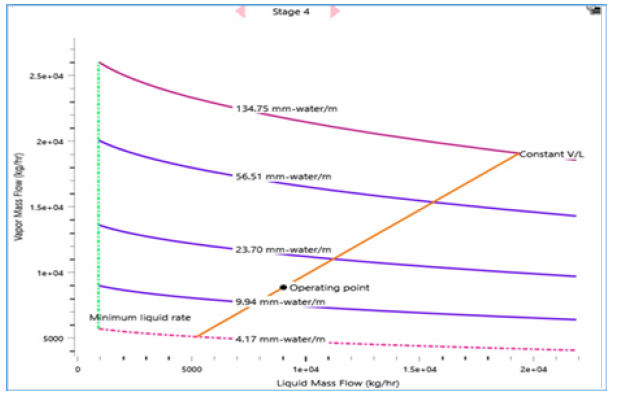
Figure 18:Hydraulic profile of the process water feed stage.
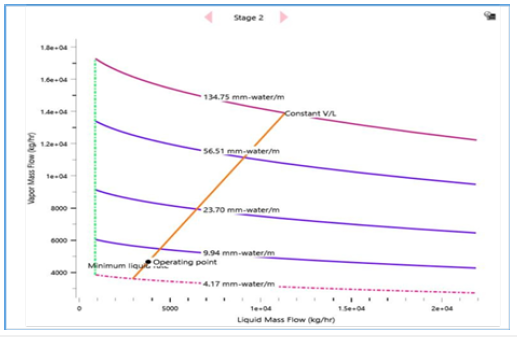
Figure 19:Vapour flow distribution along column stages.
Figure 20:Liquid flow distribution along column stages.
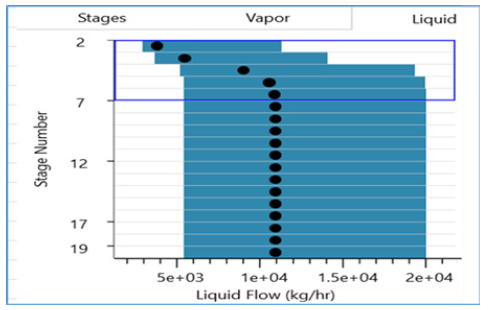
Table 6:Modelling results of the reactive system.

Table 7:Comparison of RD modelling results between this work and previous studies.

Table 8:Hydraulic analysis of MEG RD rating model.

Experimental Validation
To verify the modelling results and accuracy of modelled process parameters, an experimental validation on a reactive system for MEG synthesis is carried out. A different validation approach needs to be taken because of the limited availability of a small-scale operational test laboratory that is qualified for EO processing. For the comparison of modelling results with a real plant operating model, data from open access literature is applied together with validation result, as shown in Table 2. In addition, a sensitivity analysis using an offline test of the plant process optimizer with the RD column control system is conducted to determine the credible effects of the process parameters. Detailed information on the effects of key process parameters on MEG yields is provided (Figure 20). To improve the accuracy and predict the conversion and yield of the MEG reaction, the results of the plant test have modified the reactive stages as kinetic reactors. The sensitivity analysis for hydrolysis ratio generated a possible range of 12.3-15.4, at an average of 14 against the modelled result of 12.9. A temperature range of 190-280 °C along with a sufficient column pressure showed a good suitability for combined reaction water removal and glycol separation to achieve high MEG purity. This balances the reaction rate, MEG and water formation at controlled vapour pressure as indicated in Figure 21. Rapid removal of MEG from the reaction system is therefore attainable and ensures that a molar purity of 0.95 is obtained for MEG, which is the most volatile reacting product. Furthermore, this analysis has confirmed that the MEG yield would not be further increased by remaining reaction parameters such as temperature or pressure (Table 9).
Figure 21:RD column control scheme.
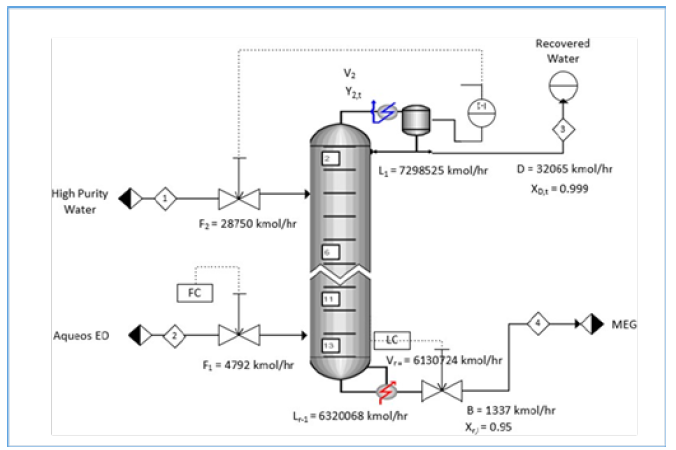
Figure 22:RD column control scheme.
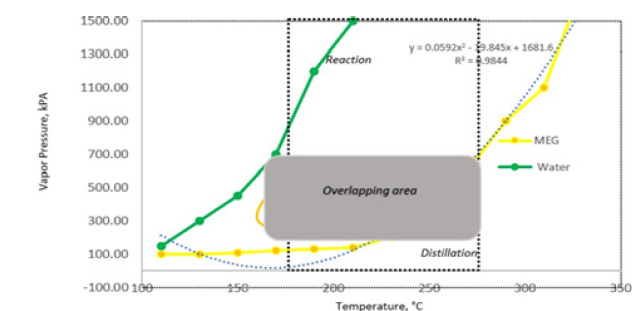
Table 9:Validation of RD process model.

Conclusion
A feasible approach to the reactive distillation for MEG production is developed with a working process model that attains higher performance. Improvements in production performance are shown that include a higher MEG yield of 95.2%, lower EO feed requirement and significantly lower energy usage for separation. This work concludes that high purity MEG product can be produced in a reactive distillation configuration that signifies major cost improvement in terms of the reduced number of processing equipment and lower operating cost benefits. Added process safety benefits are also possible in terms of lowering the possibility of outof- control reactions and EO vapour breakthroughs.
References
- Li L, Zhang Y, Peng Z, Wang C (2021) Study of organic impurities affecting ultraviolet transmittance in monoethylene glycol and their formation mechanism. Microchemical Journal 160: 105635.
- Tran A, West KJ (2021) Decarbonization options for the dutch bottle-grade PET industry. The Hague: MIDDEN.
- Akpa JG, Onuorah P (2018) Simulation and control of a reactor for the non-catalytic hydrolysis of ethylene oxide to ethylene glycol. Mathematical Theory and Modelling 8(2): 23-29.
- Ding L, Tang J, Qiao X, Liu C, Xue Y, et al. (2020) Design and analyses of an intensified column with side reactor configuration for side reactor configuration for ethylene glycol production from ethylene oxide. Chemical Engineering and Processing-Process Intensification 147: 107744.
- Steffen V, Silva EA (2018) Numerical methods and initial estimates for the simulation of steady-state reactive distillation columns with an algorithm based on tearing equations methodology. Thermal Science and Engineering Progress 6: 1-13.
- Yingjie M, Yiqing L, Xigang Y (2019) Equation-oriented optimizaiton of reactive distillation systems using pseudo-transient models. Chemical Engineering Science 195: 387-398.
- Barecka MH, Skiborowski M, Gorak A (2017) A novel approach for process retrofitting through process intensification: Ethylene oxide case study. Chemical Engineering Research and Design 123: 295-316.
- Kiss AA, Jobson M, Gao X (2019) Reactive distillation: Stepping up to the next level of process intensification. Industrial and Engineering Chemistry Research 58(15): 5909-5918.
- Nguyen V, Moonyong L (2019) Economic retrofit of reactive distillation with a total reflux design or a total boil-up design. Chemical Engineering Research and Design 145: 53-63.
- Athira GP, Riya MF (2015) Control of totally refluxed reactive distillation column using model predictive controller. International Journal of Scientific Engineering and Research (IJSER) 3(8): 31-35.
- Lijing Z, Keijin H, Ting G, Yang Y, Haisheng C, et al. (2019) Temperature inferential control of a reactive distillation column with double reactive sections. Chinese Journal of Chemical Engineering 27(4): 896-904.
- García A (2019) Multi-objective optimization of intensified processes for the purification of levulinic acid involving economic and environmental objectives. Chemical Engineering and Processing 136: 123-137.
- Aqar DY, Rahmanian N, Mujtaba IM (2019) A novel split-reflux policy in batch reactive distillation for the optimum synthesis of a number of methyl esters. Separation and Purification Technology 221: 363-377.
- Blatkiewicz M, Mißfeldt F, Smirnova I (2019) Dynamic model of batch enzymatic reactive distillation for the production of R-2-Pentyl butyrate. Industrial and Engineering Chemistry Research 58(51): 22820-22834.
- Cho M, Han M (2018) Dynamics and control of entrainer enhanced reactive distillation using an extraneous entrainer for the production of butyl acetate. Journal of Process Control 61: 58-76.
- Dai SB, Lee HY, Chen CL (2019) Design and economic evaluation for the production of ethyl lactate via reactive distillation combined with various separation configurations. Industrial and Engineering Chemistry Research 58(15): 6121-6132.
- Fonseca JD, Latifi AM, Orjuela A, Rodríguez G, Gil ID (2020) Modelling, analysis and multi-objective optimization of industrial batch process for the production of tributyl citrate. Computers and Chemical Engineering 132: 106603.
- Li G, Wang C, Guang C, Zhang Z (2020) Energy-saving investigation of hybrid reactive distillation for n-butyl acetate production from two blending feedstocks. Separation and Purification Technology 235: 116163.
- Li X, Wang R, Yan Y, Zhao R, Li H, et al. (2019) Ethylene glycol recovery from 2-Ethyl-1,3-dioxolane hydrolysis via reactive distillation: Pilot-scale experiments and process analysis. Industrial and Engineering Chemistry Research 58(45): 20746-20757.
- Huang W, Li H, Wang R, Li X, Gao X (2017) Application of the aldolization reaction in separating the mixture of ethylene glycol and 1,2 butanediol: Kinetics and reactive distillation. Chem Eng Process 120: 173-183.
- Jana AK, Banerjee S (2018) Neuro estimator-based inferential extended generic model control of a reactive distillation column. Chemical Engineering Research and Design 130: 284-294.
- Novita FJ, Lee HY, Lee M (2018) Reactive distillation with pervaporation hybrid configuration for enhanced ethyl levulinate production. Chemical Engineering Science 190: 297-311.
- Pan Q, Gao L, Li J, Yan J, Zhang L, et al. (2020) Process optimization and plant-wide control for producing 1,3-dioxolane from aqueous formaldehyde and ethylene glycol. Separation and Purification Technology 236: 116235.
- Yamaki T, Matsuda k, Na-Ranong D, Matsumoto H (2018) Energy-saving performance of reactive distillation process for TAME synthesis through multiple steady state conditions. Chemical Engineering and Processing 130: 101-109.
- Zhang Q, Liu Y, Guo T, Xu P, Wang Y (2019) Comparison of the improved processes for amyl acetate by reactive distillation in different operating modes. Asia-Pacific Journal of Chemical Engineering 14(6): e2373.
- Li X, Wang R, Na J, Li H, Gao X (2018) Reversible reaction-assisted intensification process for separating the azeotropic mixture of ethanediol and 1,2-butanediol: Reactants Screening. Ind Eng Chem Res 57(2): 710-717.
© 2024 Jofry Othman. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






