- Submissions

Full Text
Progress in Petrochemical Science
Eco-Insights on Hydrocarbon Bioremediation
Maria Fátima Nunes Serralha and Ana Cláudia de Sousa Coelho*
Polytechnic Institute of Setúbal, Escola Superior de Tecnologia do Barreiro, Department of Chemical and Biological Engineering, Portugal
*Corresponding author:Cláudia de Sousa Coelho, Polytechnic Institute of Setúbal, Escola Superior de Tecnologia do Barreiro, Department of Chemical and Biological Engineering, Portugal
Submission: April 05, 2024;Published: April 19, 2024

ISSN 2637-8035Volume6 Issue2
Abstract
Microorganisms are ubiquitous in the biosphere, influenced by both their surrounding environment and the biotic and abiotic factors of ecosystems. Contaminated areas often harbor species adept at utilizing pollutants as nutrients, thus enhancing their resilience to environmental pressures. Identifying and characterizing these species can significantly benefit bioremediation processes. To contribute to this understanding, bioremediation experiments were conducted in Barreiro, a pivotal industrial hub in Portugal during the 20th century. Soil samples from two distinct locations were analyzed: one near Barreiro city, impacted by various anthropogenic activities leading to increased fuel pollutants and the other in a protected wooded area designated for recreational and educational purposes. All identified hydrocarbon-degrading microorganisms belonged to the Pseudomonas genus. Bioremediation assays isolated bacteria from pure colonies, compared with mixtures of all bacterial morphotypes capable of growth in the pollutant’s presence. Consistently higher bioremediation rates for gasoline and toluene were observed using mixed cultures. Effective degradation-capable bacterial strains were identified in both locations, demonstrating bioremediation potential. Mixed bacterial cultures exhibited superior degradation rates, underscoring the importance of microbial diversity for bioremediation effectiveness. Also, the composition and bioremediate activity of microbial communities change in response to hydrocarbon concentration were analyzed, providing insights into the resilience of microbial communities to environmental stressors and their capacity to adapt to contaminated conditions. These findings emphasize the importance of in-depth, on-site investigations to elucidate the interplay between native microbial communities and pollutant degradation potential in soil.
Keywords:Soil bioremediation; Hydrocarbonoclastic microorganisms; Barreiro municipality; Pseudomonas
Introduction
Despite all the changes that are happening in the world and all the concerns to decrease the carbon footprint, fossil fuels are still the driver of economic growth. The low polarity of these compounds, reflected in lipophilic and hydrophobic properties, leads to high rates of persistence and ecotoxicity, being the hydrocarbons considered one of the main pollutants on the planet, causing adverse effects on the abiotic and biotic components of the Earth’s ecosystems [1,2]. The petroleum industry and associated storage, transport and trade of oil are still one of the biggest causes of contamination of extensive areas of soil and water [3]. With a more local impact, the transport spills on land appear in the 2021 European Indicator Assessment as the fifth key source of soil contamination [4]. The pollutants associated with this source of soil contamination are Petroleum Hydrocarbons (PHCs), which comprise oil and the products refined from oil, such as gasoline and diesel fuels, solvents and lubricants, among other chemical products. PHCs have a broad range of physicochemical characteristics, depending on the amount and the arrangement of carbons in their molecular structure [5,6]. Their complexity is very relevant to determining their impact in the ecosystems, since PHCs’ potential for distribution and persistence changes significantly with their chemical structure. Short-chain hydrocarbons can be more easily transported through the environment and therefore can spread over larger areas, unlike long-chain and branched hydrocarbons that do not have this mobility but are more persistent in the environment. Also, the ability of PHCs to interact with biological membranes and eventually harm organisms is dependent on their physical and chemical properties, which control their bioavailability and toxicity [7,8].
In the specialized literature, it is strongly reported that contaminated soils have changes in their microbiological, chemical and physical properties, with repercussions on groundwater, aquifer systems and air quality. It is also common to find a significant association between soil, water, food and health since pollutants can accumulate at various levels in food chains and disrupt physiological or biological processes in several species [9-12]. The relevance of this correlation between soil and health is strongly present in the concept of “healthy soils”, introduced by the European Union as an ambitious condition to be achieved by 2050 [13]. The environment contaminated with these nonpolar pollutants also naturally contains organisms capable of resisting their presence and capable of metabolizing these harmful contaminants into harmless or non-toxic substances. Natural bioremediation has always accompanied human activities. The technologies based on bioremediation processes are one of the most promising methods for the decontamination of environments, gaining flow globally due to their cost-effective and green approach, being aligned with the sustainability path [14-17].
There is a multiplicity of microorganisms with bioremediation potential, which can metabolize and not suffer harmful effects due to the presence of the contaminating agent in their habitat. These different species constitute specific microbiomes with distinct properties, functions and interactions with their environment. The development of microbial biotechnology and high-throughput sequencing technology has already identified several functional bacterial strains from environments contaminated with PHCs [18-22]. The high potential of hydrocarbon metabolization is related to microorganisms’ degradation pathways, associated with numerous intracellular and extracellular enzymes able to convert complex pollutants into carbon and energy sources [23- 25]. Knowledge about these microorganisms, their metabolic pathways and enzymes enables technical approaches needed to optimize bioremediation to its full potential. Areas where there is, or has been, strong industrial activity have been identified as key indicators for pollution increase and instability of ecosystems. This fact is not only related to the industrial activity they carry out but also to all the transport of raw materials and manufactured goods, like rail and port complexes associated with it, that promotes the formation of oil spill areas [2,26,27].
The municipality of Barreiro is located on the south bank of the Tagus River estuary, facing Lisbon. It was from the early 1900s, with the implementation of the Industrial Union Company (CUF), until the end of the 20th century, it was one of the most important Portuguese industrial cities. However, an intense process of deindustrialization started in the 1970s, led to a requalification of the Municipality that ceased to be an important industrial powerhouse in Portugal and became a municipality with a reasonable concentration of small companies [28]. Nowadays, Barreiro is a post-industrial city that seeks urban restructuring and where several urban area requalification projects have taken place. However, the memory of the industrialization period is still present and in some places of the city still visible. This study aims to enhance our comprehension of the effects of soil contamination on soil biota, specifically in the diversity of cultivable hydrocarbonoclastic microorganisms found at two sites, one of them experiencing environmental disturbances. It also investigates the degradation rates of hydrocarbons achieved using either a single type of colony or mixtures of all identified colonies on-site capable of degrading the pollutant in question.
Experimental
Experimental studies were carried out, referring to the verification of the existence and characterization of hydrocarbonoclastic microorganisms in soil samples obtained in two different areas, one located on the outskirts of Barreiro city, in a requalified beach area close to a Tagus river terminal (Praia Alburrica, GPS:38.656527, -9.087775) and the other in a protected wooden area, 7km from the same city, where only leisure activities and environmental education take place (Mata da Machada, GPS: 38.606133, -9.025713). In October 2022, two independent sets of ten top subsamples (0-15cm) depth, were obtained in the two chosen locals using a simple random system (zig-zag sampling pattern). For each site, the soil core was selected separately, randomly and independently of previously drawn units. The soil subsamples collected at each site were mixed and homogenized to obtain a representative single composite sample [29]. Due to the significant variability of PHCs (Petroleum Hydrocarbons), three simpler representative compounds that are always present in fuels were selected: gasoline (typically a blend of linear hydrocarbons containing 5 to 10 carbon atoms), hexane (a linear hydrocarbon with 6 carbon atoms) and toluene (also known as methylbenzene, representing aromatic compounds).
For microbial selection, parallel sets of flasks with 1g of each composite sample were prepared in 100mL freshly prepared Bushnell Haas Mineral Salt medium (BH) and placed at 25 °C with continuous stirring at 180rpm for 5 days. After the selection of the hydrocarbonoclastic microorganisms and before all the other growth studies, an enrichment step was done transferring the microbial population to Luria-Bertani medium (LB) to an overnight growth at 25 °C and 180rpm. The monitoring of bacterial growth (25 °C with 180rpm for 30 hours) in BH containing 1.0% (v/v) and 0.2% (v/v) of the corresponding pollutant as the unique carbon source, was performed by reading the Optical Density (OD) at 600nm in a microplate reader (BMG LABTECH). In these assays, the initial OD600nm was adjusted to 0.1. The cultivable bacterial colonies were obtained in BH agar plates supplemented with 0.2% and 1.0% (v/v) of PHCs and incubated at 25 °C for 7 days. To ensure unbiased data, morphology characterization was performed in plates with 5 to a maximum of 15 colonies. The purified strains were used in Gram Test and bioremediation studies. The identification of Pseudomonas was done using a selective medium Pseudomonas CN Agar Base EN ISO 16266 (Dehydrated Culture Media) for microbiology (ITW Reagents).
The colonies that showed fluorescent under a Wood’s lamp and positive oxidase test were considered Pseudomonas aeruginosa, colonies without fluorescent pigment but a positive oxidase test and fermentation of glucose on Purple Glucose Agar (REF 401970) were confirmed to be Pseudomonas aeruginosa. Other colonies, showing a positive oxidase reaction but the absence of glucose fermentation, were considered Pseudomonas spp. The experimental bench‐scale bioremediation rates for the three PHCs were determined using the redox indicator 2,6-Dichlorophenolindophenol (DCPIP) after 5 days of incubation at 25 °C and 180rpm [30]. Five replicates were done for each biodegradability study and the mean standard deviation values were calculated. The statistical analysis was done by independent t-test. As a null hypothesis, it was considered that the rates of bioremediation do not differ between pure cultures and bacterial culture mixtures. A p-value of <0.05 was the significance level for declaring statistical significance.
Results
Microbial growth curves
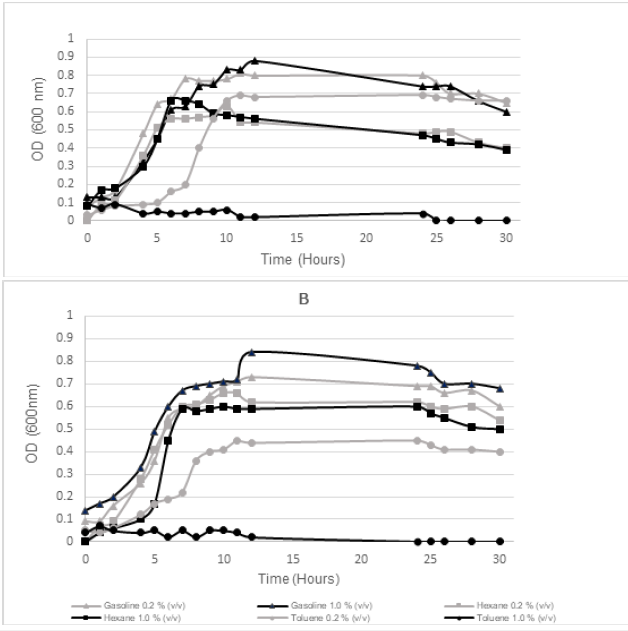
The growth curves (Figure 1) show that in all conditions studied, the maximum OD achieved remained below 1, being the stationary phase reached after 5 to 12 hours. The more relevant difference was observed during the microbial growth in the presence of toluene, with longer lag phases observed at 0.2% and with no growth at all in the presence of 1.0% of this compound. This fact indicates the higher toxicity of toluene for these bacteria consortiums. The differences in growth dynamics of Mata da Machada (Figure 1A) microorganisms, are more related to the maximum OD reached, being the specific growth rates (μ) very similar to each other, having an average value of 0.12506 ± 0.01628(h-1). However, there is greater variability in growth rates obtained during Praia da Alburrica (Figure 1B) microbial growth, which varied between 0.0836h-1 in the presence of 0.2% toluene and 0.210h-1 in the presence of 1.0% hexane.
Figure 1:Consortia growth curves in mineral medium BH with 0.2 or 1.0% (v/v) of gasoline, hexane and toluene A: Mata da Machada; B: Praia da Alburrica.
Cultivation and identification of bacterial colonies
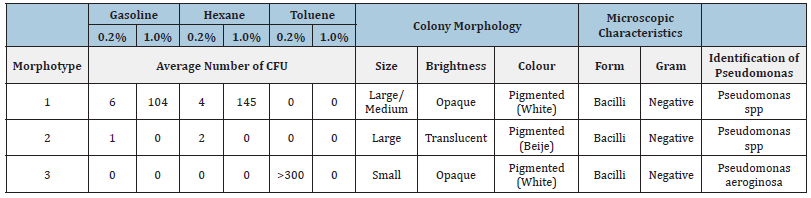
The colony morphology, or morphotype, represents a group of bacteria grown from a single cell on an agar surface, that is, it represents a Colony-Forming Unit (CFU) with a typical colonial pattern. The morphotypes that will be presented were observed at least in 3 of the 5 replicates. All the visible colonies showed a circular shape and smooth structure, raised in relation to the culture medium and presented entire margins. The characteristics that allowed us to distinguish the morphotypes of the hydrocarbonoclastic colonies were size, colour and brightness (Table 1). The soil sample from Mata da Machada recorded a generally higher number of Colonies Forming Units (CFU) in the presence of gasoline and hexane. In their morphological characterization, it was possible to identify some colonies capable of producing pigment with different varieties of colours, such as yellow, orange and pink (Table 1). Although it is documented that there are several colonies capable of synthesizing pigments, especially under adverse environmental conditions, for the genus Pseudomonas only the possibility of synthesis of the pigments pyoverdine with a yellowish green colour and pyocyanin with greenish-blue-colour is reported [31,32]. The culturable colonies corresponding to the composite sample of Praia da Alburrica (Table 2), present a lower morphological diversity (only three morphotypes). Toluene at 0.2% seems to exert environmental pressure by selecting only one morphotype of bacteria able to metabolize this compound. This competitive advantage was also verified when analyzing the soil sample from Mata da Machada (Table 1), where Pseudomonas aeroginosa CFU appeared in a much higher number in the presence of toluene.
Table 1:Number of CFU, morphological characterization, Gram test and identification of Pseudomonas species, of the cultivable Mata da Machada bacterial colonies obtained in BH agar plates, supplemented with 0.2% and 1.0% (v/v) of gasoline, hexane and toluene as the only carbon source.
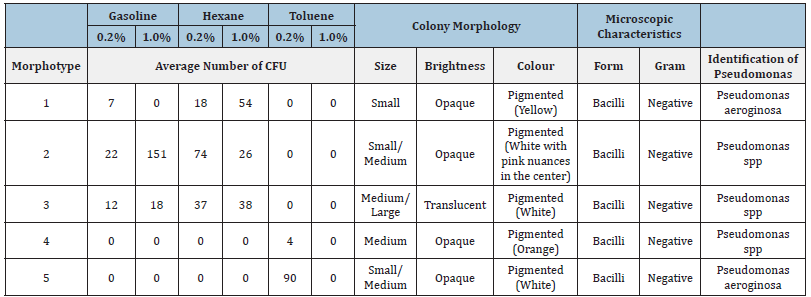
Table 2:Number of CFU, morphological characterization, Gram test and identification of Pseudomonas species, of the cultivable Praia da Alburrica bacterial colonies obtained in BH agar plates, supplemented with 0.2% and 1.0% (v/v) of gasoline, hexane and toluene as the only carbon source.
Bioremediation rates
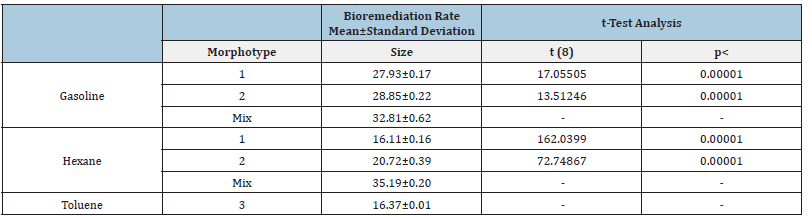
The DCPIP assay allowed the confirmation of the hydrocarbon’s degradation capacity of the selected colonies. The biodegradability rates calculated are clustered around the mean, showing a coefficient of variation (standard deviation/mean) lower than 0.01 (Table 3,4). The average biodegradation level after five days can be arranged in descending order as follows: gasoline, hexane and lastly toluene. The only exception was the biodegradation value of hexane obtained in the mixed culture of colonies grown with this compound as the only carbon source, selected at Praia da Alburrica (Table 4). The rates obtained for the bioremediation of each pollutant, using the pure cultures, do not present a noteworthy variation between each other. When comparing these values with those obtained from bioremediation conducted by mixing colonies, a notable disparity emerges, consistently indicating higher success in bioremediation employing bacterial consortia. To assess the significance of this observation, a statistical analysis was conducted using the independent t-test to compare the averages of bioremediation rates achieved with pure colonies versus those with mixed colonies. The null Hypothesis (H0) posited that the genuine disparity between the mean bioremediation rates of these two groups is zero. The computed t and p-values, at a significance level of 0.05 and 8 degrees of freedom, are additionally presented in Table 3 & Table 4. The obtained results lead to the conclusion that there is a significant difference between the mean bioremediation rates of the compared groups.
Table 3:Bioremediation rates and values of the independent t-test statistical analysis, obtained for the cultivable hydrocarbonoclastic colonies present in the soil sample from Mata da Machada.
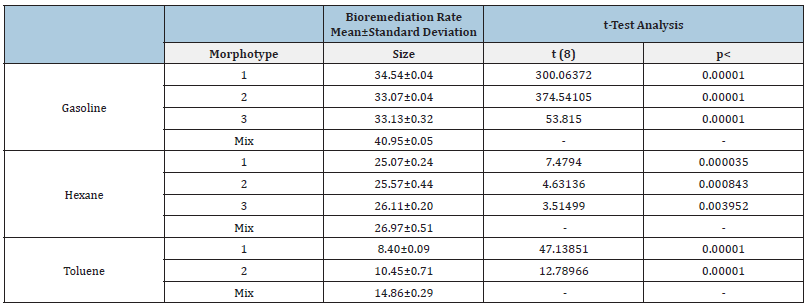
Table 4:Bioremediation rates and values of the independent t-test statistical analysis, obtained for the cultivable hydrocarbonoclastic colonies present in the soil sample from Praia da Alburrica.
Discussion
Through the enrichment and microorganism’ selection methodologies, valuable insights into the hydrocarbon-degrading bacteria present in soil samples from different areas within the Barreiro Municipality were obtained. Despite the absence of major differences in microbial richness between the two areas, the findings underscore consistent trends in microbial community composition and bioremediation potential. The identification of the genus Pseudomonas in both locations underscores its well-documented hydrocarbonoclastic capacity [18,22,33] offering promising avenues for bioremediation strategies. Notably, the observed decrease in evenness in locations with higher human activity emphasizes the impact of anthropogenic factors on microbial community dynamics. Furthermore, the higher bioremediation rates observed in assays incorporating all identified colonies underscore the importance of considering the entirety of microbial communities in remediation efforts. The limitations posed by the inability to culture many microorganisms using standard techniques and the potential for multiple strains to exhibit similar morphotypes increase the challenges in accurately assessing species representativity, underscoring the complexity of microbial communities in polluted soil. Moving forward, further research in this area will be instrumental in deepening our understanding of these extensive microbial communities and dynamics within the ecosystem. Moving forward, further research in this area will be instrumental in deepening our understanding of the extensive microbial communities in polluted soil, ultimately facilitating the development of more effective and sustainable bioremediation strategies tailored to specific environmental contexts.
Regarding PHCs biodegradation, data obtained is in accordance with previous research which reported that alkanes are the most biodegradable of the petroleum hydrocarbons [28,34] compared to the aromatic or naphthenic compounds and that gasoline exhibited a high intrinsic biodegradability [35]. In both analyzed samples, toluene, an aromatic hydrocarbon, was the hydrocarbon that most limited the growth of microorganisms, especially at a concentration of 1.0%, where no colonies were able to grow. This fact may be related to its chemical structure, being an aromatic hydrocarbon while the other tested pollutants are linear, or with the fact that being toluene a volatile hydrocarbon, the soil is not naturally enriched with microorganisms capable of biodegrading it. The last argument agrees with the fact that only one type of colony had been capable of surviving with toluene as a carbon source. Laboratory-based biodegradation tests simulate natural processes, however, it is not possible to precisely reproduce the natural biodegradation due to the inequality of several environmental factors, such as physicochemical characteristics of the soil, environmental conditions and the microbial populations involved in the biodegradation. Notwithstanding the use of autochthonous microorganisms in these studies is an advantage since they are better adapted not only to the possible presence of the pollutant but also to all the abiotic and biotic parameters of the environment [15,36].
The increased bioremediation when using the sample of microorganisms selected from the soil seems to indicate that this process is more effective using mixed cultures and may reveal positive interactions between microorganisms. This data has already been evidenced by other authors that highlight the importance of bacterial consortia to increase bioremediation rates [22,23,33,37]. Moreover, it’s important to recognize that petroleum and its derivatives are natural compounds susceptible to bioremediation. However, the overarching issues surrounding the Sustainable Development Goals (SDGs) pertain to their excessive exploitation, production and use patterns and the significant quantities deposited in the environment. This study underscored the ecosystems’ capability to degrade hydrocarbon compounds. However, it also highlighted the challenge in the survival of hydrocarbonoclastic microorganisms, particularly in the presence of chemically complex compounds like toluene. Despite the resilience of ecological systems to various natural and humaninduced factors, this study also revealed that this resilience has its limits.
Conclusion
Solid oxide fuel cells and electrolysers stand as beacons of hope in our quest for a sustainable future. Beyond their scientific intricacies, they embody a convergence of innovation and belief, where the boundaries between science and spirituality blur. As we unravel the mystique of these marvels of modern science, let us embrace the new perspectives, recognizing that true progress lies not only in technological advancement but also in the evolution of consciousness.
References
- Srivastava M, Srivastava A, Yadav A, Rawat V (2019) Source and control of hydrocarbon pollution. In: Ince M, Ince OK (Eds.), Hydrocarbon Pollution and its Effect on the Environment. IntechOpen, London.
- Stepanova AY, Gladkov EA, Osipova ES, Gladkova OV, Tereshonok DV (2022) Bioremediation of soil from petroleum contamination. Processes 10(6): 1224.
- Chicca I, Becarelli S, Gregorio SD (2022) Microbial involvement in the bioremediation of total petroleum hydrocarbon polluted soils: Challenges and perspectives. Environments 9(4): 52.
- Paya PA, Rodríguez EN (2018) Status of local soil contamination in Europe: Revision of the indicator “progress in the management contaminated sites in Europe”. EUR 29124 EN, Publications Office of the European Union, Luxembourg.
- Sakshi SS, Haritash AK (2019) Policyclic aromatic hydrocarbons: Soil pollution and remediation. International Journal of Environmental Science and Technology 16: 6489-6512.
- Zheng X, Oba BT, Shen C, Rong L, Zhang B, et al. (2023) Effect of the bacterial community assembly process on the microbial remediation of petroleum hydrocarbon-contaminated soil. Frontiers in Microbiology 14: 1196610.
- Koshlaf E, Ball AS (2017) Soil bioremediation approaches for petroleum hydrocarbon polluted environments. AIMS Microbiol 3(1): 25-49.
- Radice RP, Fabrizio VD, Donadoni A, Scopa A, Martelli G (2023) Crude oil bioremediation: From bacteria to microalgae. Processes 11(2): 442.
- Haque S, Srivastavac N, Pal DB, Alkhanani MF, Almalki AH, et al. (2022) Functional microbiome strategies for the bioremediation of petroleum-hydrocarbon and heavy metal contaminated soils: A review. Science of the Total Environment 833: 1-8.
- Lull C, Lidón A, Soriano MD (2022) Soil pollution education: A broad view of knowledge on soil pollution and educational activities for undergraduate students. Adv Environ Eng Res 3(2): 18.
- Silva IG, Almeida FC, Silva NM, Casazza AA, Converti A, et al. (2020) Soil bioremediation: Overview of technologies and trends. Energies 13(18): 1-25.
- Liang Y, Zhao H, Deng Y, Zhou J, Li G, et al. (2016) Long-term oil contamination alters the molecular ecological networks of soil microbial functional genes. Frontiers in Microbiology 7: 1-13.
- Panagos P, Montanarella L, Barbero M, Schneegans A (2022) Soil priorities in the European Union. Geoderma Regional 29(4): e00510.
- Bala S, Garg D, Thirumalesh BV, Sharma M, Sridhar K, et al. (2022) Recent strategies for bioremediation of emerging pollutants: A review for a green and sustainable environment. Toxics 10: 484.
- Sarkar J, Roy A, Sar P, Kazy S (2020) Accelerated bioremediation of petroleum refinery sludge through biostimulation and bioaugmentation of native microbiome. Emerging Technologies in Environmental Bioremediation 23-65.
- Nariyal M, Yadav M, Singh N, Yadav S, Sharma I, et al. (2020) Microbial remediation progress and future prospects. Bioremediation of Pollutants 187-214.
- García RR, Gohil N, Singh V (2018) Recent advances, challenges and opportunities in bioremediation of hazardous materials in phytomanagement of polluted sites. Phytomanagement of Polluted Sites 517-568.
- Oba A, John B, Garba J, Oba AJ, Veronica K, et al. (2022) Bioprospecting of hydrocarbonoclastic representative bacteria. Journal of Environmental Protection 13(6): 449-458.
- Chandran H, Meena M, Sharma K (2020) Microbial biodiversity and bioremediation assessment through omics approaches. Front Environ Chem 1: 25.
- Badienyan S, Marand AD, Hajipour MJ, Ameri A, Razzaghi MR, et al. (2018) Detection and discrimination of bacterial colonies with muller imaging. Sci Rep 8(1): 10815.
- Hazen TC, Prince RC, Mahmoudi N (2016) Marine oil biodegradation. Environ Sci Technol 50(5): 2121-2129.
- Yang Y, Wang J, Liao J, Xie S, Huang Y (2015) Abundance and diversity of soil petroleum hydrocarbon-degrading microbial communities in oil exploring areas. Appl Microbiol Biotechnol 99: 1935-1946.
- Zhang S, Merino N, Okamoto A, Gedalanga P (2018) Interkingdom microbial consortia mechanisms to guide biotechnological applications. Microbial Biotechnology 11(5): 833-847.
- Teng Y, Chen W (2019) Soil microbiomes-a promising strategy for contaminated soil remediation: A review. Pedosphere 29(3): 283-297.
- Bharagava RN, Purchase D, Saxena G, Mulla SI (2018) Applications of metagenomics in microbial bioremediation of pollutants: From genomics to environmental cleanup. Microbial Diversity in the Genomic Era 459-477.
- Gao J, Faheem M, Yu X (2022) Global research on contaminated soil remediation: A bibliometric network analysis. Land 11(9): 1581.
- Orellana R, Cumsille A, Gangas PP, Rojas C, Arancibia A, et al. (2022) Economic evaluation of bioremediation of hydrocarbon contaminated urban soils in Chile. Sustainability 14(19): 11854.
- Medeiros E (2018) Deindustrialization and post-industrial cities in Iberia Peninsula. Revista Portuguesa de Estudos Regionais 52: 37-53.
- Carter M, Gregorich E (2008) Soil Sampling and methods of analysis. In: Carter M, Gregorich E (Eds.), (2nd edn), CRC Press, Boca Raton, Florida, pp. 1264.
- Kubota K, Koma D, Matsumiya Y, Chung S, Kubo M (2008) Phylogenetic analysis of long-chain hydrocarbon-degrading bacteria and evaluation of their hydrocarbondegradation by the 2,6-DCPIP assay. Biodegradation 19(5): 749-757.
- Celedón RS, Díaz LB (2021) Natural pigments of bacterial origin and their possible biomedical applications. Microorganisms 9(4): 739.
- Vélez JM, Martínez JG, Ospina JT, Agudelo SO (2021) Bioremediation potential of Pseudomonas genus isolates from residual water, capable of tolerating lead through mechanisms of exopolysaccharide production and biosorption. Biotechnology Reports 32: e00685.
- Oba A, John B, Garba J, John KV, Allamin IA, et al. (2022) Hydrocarbon degradation competence of bacterial consortium isolated from oil polluted soil in azuabie town, port harcourt, Nigeria. Journal of Biochemistry, Microbiology and Biotechnology 10(1): 36-40.
- Chaerun SK, Tazaki K, Asada R, Kogure K (2004) Bioremediation of coastal areas 5 years after the Nakhodka oil spill in the Sea of Japan: Isolation and characterization of hydrocarbon-degrading bacteria. Environ Int 30(7): 911-922.
- Marchal R, Penet S, Serena FS, Vandecasteele JP (2003) Gasoline and diesel oil biodegradation. Oil & Gas Scienc Technol 58 (4): 441-448.
- Hosokawa R, Nagai M, Morikawa M, Okuyama H (2009) Autochthonous bioaugmentation and its possible application to oil spills. World J Microbiol Biotechnol 25: 1519-1528.
- Zhang T, Zhang H (2022) Microbial consortia are needed to degrade soil pollutants. Microorganisms 10(2): 261.
© 2024 Ana Cláudia de Sousa Coelho. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






