- Submissions

Full Text
Progress in Petrochemical Science
Review, Biodiesel Production from Basic to Advance Level Through Using Different Mechanism, Techniques, Approaches and How to Make It Commercialized
Inam Ullah Khan*
State Key Laboratory of Advanced Special Steel, Shanghai University, China
*Corresponding author:Inam Ullah Khan, State Key Laboratory of Advanced Special Steel, Shanghai Key Laboratory of Advanced Ferro metallurgy, School of Materials Science and Engineering, Shanghai University, China
Submission: December 19, 2023;Published: January 17, 2024

ISSN 2637-8035Volume6 Issue1
Abstract
Uncritical withdrawal and usage of mineral oil has reduced fossil fuel assets, causing fuel shortages and ecological deprivation. Due to fossil fuels’ environmental impact, biodiesel manufacturing has garnered interest as an alternative to Petro diesel. Biodiesel is a renewable diesel fuel made from vegetable oils and animal fats. Renewable energy seems like a good option for world energy needs, including Pakistan. Thus, a state-available feedstock-based alternative fuel must be found. Although vegetable oil can be used in diesel locomotives, using it for biodiesel production has become a major concern because it competes with food nutrition, making it difficult to defend. Thus, the search for inedible oil-yielding plant sources for biofuels has been fascinating and beneficial to the environment and food safety. Trans esterifying non-edible oil with methanol and base or strong acid catalysts make Biodiesel. Several parameters affect transesterification reaction. An optimum transesterification reaction depends on oil fatty acid composition and free fatty acid concentration. Other factors include reaction temperature, alcohol-to-vegetable oil ratio, catalyst, mixing intensity and reactant purity. The kinematic viscosity, acid value, density, water content, flash point, pour point, cloud point and cold filter plugging point of biodiesel will be determined using ASTM standards (D-6751) and EN (14214) with acceptable agreement. To determine biodiesel composition and structure, FT-IR, NMR (1H & 13C), and GC-MS will be used. ICP-OES will calculate Na, K, Ca and Mg compositions. Elemental Analyzer (EA) investigations would analyze C, H, O and N ratios. Future feedstock research should reveal production costs, technological transformation opportunities for farmers, and worldwide industrial applications. Biodiesel fuel characteristics, transesterification and the most essential variables affecting the reaction are covered in this article.
Keywords:Non-edible seeds; Transesterification; Base catalysis; Environmentally friendly; Alternate energy; Biodiesel
Introduction
Energy is one of the key efforts for growth factors in all sectors, including agriculture, industrial services and transportation. For decades, energy has been a significant factor in developing the national and global economy improvisation. The demand for energy in the world, especially petroleum energy, is growing exponentially. Therefore, future energy supply and devolution have become a major global problem. Another primary concern of the world is environmental degradation or climate change, such as global warming. Throughout the first pledge phase, the “Kyoto Protocol” goal was to reduce the emissions of greenhouse gases of the corresponding 37 industrialized countries by a normal of 5% from 1990 stages. During the second commitment period, the contracting parties promised to reduce greenhouse gas emanations by at slightest of 18% from 1990 level over eight years retro since 2013 to 2020; though, the configuration of the contracting festivities to the contract in the subsequent commitment retro is the same as the paramount commitment only the period is different.
Biofuel is such a unique and substitutes fuel that can solve the above problems on a large scale. The supreme communal technique used to convert plant oils, animal fats and edible oil into combustible/ flammable liquid oils, namely Biodiesel, is a transesterification reaction. After the process is completed, most of the water-soluble impurities will remain in the biofuel. The effectiveness of biofuel depends mainly on the purity of the final product and whether it is completely free of particles or contaminants. For the best results, the dirt and impurities must be removed. Traditionally, wet cleaning technology has been used for purification and cleaning. Still, due to environmental problems related to wastewater treatment, it is necessary to use technologies to make the process more environmentally friendly.
This chapter first briefly introduces Biodiesel’s history and why Biodiesel is needed as a distinct alternative fuel; raw materials can be used for production, including the transesterification reaction method. The impact of impurities on biodiesel performance is also discussed, and various strategies can be used to remove biodiesel impurities. To meet ASTM/EN/IS standards, it is necessary to characterize Biodiesel using multiple analytical performances such as Gas Chromatography (GC), Inductively Coupled Plasma Spectroscopy (ICP-OES) and chemical methods. Lastly, it is required that Biodiesel should not only comply with ASTM/ EN standards, but its engine performance should also be similar to diesel.
Future Assets for Future Energy
Mineral oil or fossil fuel is used faster than it is produced. Insufficient quantities or unreasonable prices for petroleum fuel products are a significant concern for everyone. The most likely approach to elucidate these complications is to practice unusual alternate energy fuels [1]. It has been established that plant oils are a promising fuel since their possessions are comparable to diesel and can be straightforwardly, efficiently and renewably produced from plant crops [2]. Biofuels, especially Biodiesel, has the great potential to replace petroleum diesel [3]. As we all know, biofuels have many benefits over remnant fuels, such as sustainability, biodegradability, low greenhouse gas emissions, local improvement, communal and agricultural progress and the provision of safe fuel supply. In addition, replacing petroleum diesel with Biodiesel can diminish the accumulation of carbon dioxide and supplementary greenhouse gases [4].
Biodiesel; An Option of Alternative Fuel
As an unconventional to diesel petroleum, the concept of biofuel has become increasingly important worldwide due to its excellent fuel quality, reducing emissions, sustainability and biodegradability [5]. Nowadays, people/ human beings are so dependent on fossil fuels that they cannot imagine a world without petroleum. Petroleum fuel is inadequate and its revenues millions of years to form [6]. There is no doubt that various renewable and sustainable energy sources have been developed worldwide, such as solar power energy, wind power energy, hydroelectric power and geothermal energy. Still, currently, people believe that biofuels, especially Biodiesel, play a vital role in transportation. Similarly, the assortment of possible rare ingredients for biofuel manufacture, their accessibility and the price are also imperative factors requiring extensive research. Today, as global fossil fuel production is impending its top, billions of tons of carbon released into the air, comprehensive global warming, the threat of climate change and significant complications to developing other traditional energy sources must be taken due attention to developing countries, too relevant and feasible environmentally friendly renewable energy technology. Is, in this case, biofuels, mainly; Biodiesel, are known to be an essential part of several upcoming energy circumstances. In recent times, biofuel was formed from palatable raw materials such as corn, canola, and additional plant oils. Though, in many underdeveloped and developing countries, the use of the above raw materials has created many problems related to food insecurity and safety. Therefore, scientific communities worldwide should be forced to focus on their research work on Biodiesel raw materials from non-edible sources, which directly meet food safety and security requirements.
Definition of Biodiesel
Figure 1:Share of green growth ODA by DAC recipient country region.

Biodiesel is defined as “monoalkyl esters of long-chain fatty acids originate from various plant oils and animal fats under certain specified conditions.” It is obtained through a biochemical procedure termed transesterification and can be cast-off straight in diesel locomotives or assorted with petroleum diesel deprived of any modification [7-10]. The American ASTM Standards Organization originally described Biodiesel as “biofuel is a compound of fatty acid long-chain monoalkyl esters found in renewable biomass resources and castoff in diesel locomotives [7,8] in terms of stoichiometry, a single spy of triacylglycerol retorts through three spies of alcohol, resulting in a single mole of glycerol and three spies of Fatty Acid Methyl Ester (FAMEs). The whole procedure is completed in three consecutive ways (Figure 1).
The Historical Background of Biodiesel Consumption
Since ancient times, plant oil has been used as a substitute for Petro diesel fuel. Before the energy crisis in the late 1970s, it was well-known that German scientist “Rudolf Diesel” had a keen interest in renewable and sustainable biofuels. However, the history of vegetable oil in the form of different alternative petroleum diesel is often inconsistently cited because this oil is incompatible with the ideas and statements of the diesel itself. Therefore, starting with the term diesel in his book “The” Die Entstehung des Diesel motors “means the development of Diesel engines” [11], it is appropriate to begin the history of biofuels, i.e., the development and construction of diesel engines. Rudolf Diesel made it clear in the above statement that from a thermodynamic perspective, he has achieved success in the construction of a diesel engine. The primary purpose of this maritime project was to make an easy use, efficient and reliable engine. In this case, it’s generally believed that diesel has been developed as an engine; in particular, the use of vegetable oil is considered inappropriate [7]. During the Paris World Expo in the 1900s, with the help of the French Otto company, a small diesel engine ran using peanut oil. Its performance is so good that only some of them know about this unexplained thing. Initially, the machine was designed for petroleum. In this temptation, it is run and dominated by vegetable oil, its internal structure and composition have not changed and the essential characteristics of, i.e., the fuel consumption and heat output are the same as petroleum diesel [12].
Later, vegetable oil was used as an emergency fuel in World War II. Brazil is a leading oil producer and the exports of cottonseed oil were banned for use as alternative fuels [13]. Argentina also reported on the commercial development of vegetable oil [14]. In this way, the Chinese people produce alternative more diesel and lubricating oil by cracking various types of vegetable oils [15,16]. However, during World War II, cracked vegetable oil was installed in bulk quantities due to fragmentation [15]. On the subcontinent, people produce a lot of vegetable oil as an alternative fuel for the local market [17]. Due to the easy availability of oil in the local market, Indian research on vegetable oil for biofuel production was blocked entirely [18]. At that time, the Japanese were using refined soybean oil as a substitute energy fuel [19]. Recently, Biodiesel is resultant from an assortment of biomass resources, comprising animal fats, plant oils, used fried oils and even soaps raw materials. In general, future prospects for Biodiesel are based on various factors, including economic factors, geographic and environmental factors, climatic conditions and market value.
Advantages of Biodiesel (A Clean-Burning Alternative Fuel)
Easy to use: Biofuel can be cast-off in present locomotives, vehicles, cars and groundwork with almost no modifications. Biodiesel can be compelled, stowed and scorched just like fossil diesel and can only be castoff purely or mixed with Petro diesel fuel at any proportional rate. The energy and fuel economy of using biofuel is comparable to that of diesel fuel, and it can be combined with diesel fuel to achieve year-round operation and performance.
Strength and performance
The degree to which the fuel delivers the appropriate lubrication for its lubricity. The Low lubrication of Petro diesel fuel may cause premature failure of vaccination system components and reduce presentation and efficiency. Biofuel offers outstanding lubrication for the fuel inoculation arrangement.
Emissions and greenhouse gas reduction
Compared with petroleum diesel, Biodiesel can significantly reduce carbon monoxide, particulate matter, unburned hydrocarbon and sulfate emissions. In addition, compared with petroleum diesel, Biodiesel can reduce the emission of carcinogenic chemicals by up to 85%. When blended or combined with petroleum diesel, this emissions reduction is usually proportional/ equal to the amount of Biodiesel in the blended compound.
Grown, produced and distributed locally
Globally, energy security has become a hot topic for governments and civil society. Without a stable and affordable energy supply, a country’s economy will come to a standstill; the fuel is not only needed for transportation but also crucial as an energy/fuel source for power plants and factories or the heating of houses. Biodiesel manufactured using locally available raw materials can reduce the country’s dependence on the imports of expensive finished products.
Toxicity, biodegradability, safety and recycling
The occasional spill of petroleum fuel occurs within and around the establishment of plant processing and affects plants, animals and humans. The toxicity and biodegradability of Biodiesel are lower than that of Petro diesel and they are less harmful to the environment.
Economic development
The resources needed for biodiesel production can be found locally, so domestic production of Biodiesel can bring a series of economic benefits to the local community by creating more jobs opportunity, providing tax incentives/ benefits for producers through various government plans programs, and generating local income generation, manufacturers of raw materials and refiners.
Viability of biodiesel production
With so many raw materials for biodiesel production, biodiesel processing plant operators with many green industries have a competitive advantage. This is because its industries are less sensitive to price fluctuations, as they can choose from the range of readily available raw materials used to produce Biodiesel.
Therefore, this brings a win-win situation for all stakeholders in the industry. Biodiesel manufacturers, former and growers, can make a profit and continue to operate in a thriving market with flexible consumables raw materials.
Issues with Biodiesel Production
With the production of Biodiesel, there are specific problems related to raw materials and processes.
Raw material related issues
Two essential factors related to the quality of raw materials will affect biofuel making, namely the presence of moisture and the content of high free fatty acid in the oil. The existence of free fatty acids and water content during the base-catalyzed transesterification reaction will decrease biofuel revenue and prevent downstream departure and cleansing. Therefore, it is best to use low FFA and oils with low water content and while all other substances (such as alcohol) should be essentially water-free.
High free fatty acid content of oil
The choice of biofuel manufacture process hinges on the free fatty acid content in the feedstock [20], as a fragment of the FFA retorts with the catalyst to form soap, as shown in Figure 2. Consequently, the catalyst component has been consumed and is no longer obtainable in the reaction of transesterification. This leads to a decrease in reaction efficiency. The extreme amount of free fatty acids adequate in an alkali-catalyzed system is not as much of more than 2%, somewhat fewer than 1%. Ma et al. recommended that the FFA gratified in oils must be as low as conceivable, less than 0.5% [21].
Figure 2:FFA effects in the transesterification (cited from e-education.edu.psu).

Moisture content of oil
The moisture content in the feedstock/oil should be as low as possible. If oil with excess water is used as raw material, the water will hydrolyze the oil into free fatty acids, resulting in an upsurge in the free fatty acids content in the oil. In term, this will continue to lead to the formation of a large amount of soap. Therefore, the feedstock should be dried before adding it to biodiesel production [22]. Acid-catalyzed transesterification of oil with high free fatty acid gratified is also impossible because the reaction takes a longer time to complete. The amount of alcohol required is also high. In the circumstance of exceedingly acidic crude resources, ester exchanges [6,23] should be performed as the preliminary/principal stage to decrease FFA gratified to the above value by esterification and then a base-catalyzed transesterification reaction. Specific methods that use extraordinary free fatty acid feedstock usage this thought to “improve” free fatty acids from the feedstuff for discarding in the esterification unit are separate. The acid can be added to the raw material, and the resulting soap is removed using a centrifuge. This is called caustic peeling. During a caustic stripping process, the soap loses some triglycerides. The detergent combination can be acidified to recuperate fatty acids and misplaced oil in an isolated retort tank.
The refined oil will be dried out and directed to the transesterification unit for supplementary treating. Instead of wasting free fatty acids removed in this way, it is better to convert them into methyl esters using the acidification method. Cheaper ingredients (such as tallow or yellow fates) usually contain great Free Fatty Acids (FFA). The average range for grease and yellow fat is FFA≤15%. Direct esterification of an extraordinary free fatty acid feed supply needs to eliminate water in the reaction; otherwise, the reaction will be extinguished impulsively. In addition, higher doses of alcohol and FFA are required, usually between 20:1 and 40:1. Depending on the method used, straight esterification may also necessitate a hefty amount of acid catalyst. The esterification reaction of FFA and methanol yields water as a by-product, which requisite be detached; nevertheless, the consequential assortment of esters and triglycerides can be cast-off directly in predictable alkali-catalyzed systems. It can be separated as a water mixture of methanol by vaporization, sedimentation, or centrifugation. A counter-current continuous flow system will continue to wash water through the discharged acidic methanol stream [8].
Process Related Issues
The various problems associated with the biodiesel production process are different alcohol, catalyst, the proportion of alcohol to the catalyst, reaction time and temperature.
Type of alcohol used
Alcohol that can be cast-off in biofuel making is short-chain alcohol, containing methanol, ethanol, butanol and amylic alcohol [24-28]. Due to their low cost and performance, the utmost extensively used alcohol is methanol (CH3OH) and ethanol (C2H5OH). Although methanol is highly toxic, it is generally more popular than ethanol since its biofuel-making expenditure involves modest equipment. Excessive alcohol can be improved at a lower rate and achieve a sophisticated reaction rate. Compared with methanol, the emulsion formed during the reaction is more prone to instability than ethanol [29]. Ethanol on ethanolysis has decomposed to create a very stable emulsion, and it isn’t easy to separate the phases during biodiesel purification.
Alcohol to oil ratio
The volume ratio of alcohol to oil is one of the main variables in the transesterification process. The stoichiometric measurement ratio requires 1 mole of oil to react with three moles of alcohol to obtain 3 moles of Fatty Acid Methyl Ester (FAME) and 1 mole of glycerin. However, because the reaction is reversible, excess/ more alcohol as a reactant will move the balance to the right side of the equation, thus increasing the product value. Although a high alcohol/oil ratio will not change the FAME’s performance, it will make the separation of Biodiesel from glycerol more problematic as it will increase the solubility of the former in the latter. Usually, 100% excess alcohol is consumed in practice, i.e., 6 moles of alcohol per mole of oil is used for base catalysis, and 30:1 for acid catalysis [30].
Catalysts
The catalyst used for the transesterification of triglycerides may be homogenous [31-34], heterogeneous [35-37], or by enzymatic [38-41] alkaline catalysts including Sodium Hydroxide (NaOH), Potassium Hydroxide (KOH) and its associated alkoxide (for example, sodium methoxide or ethoxide). There are many references to basic catalyst observations in the scientific literature [42]. Acid catalysts include sulfuric acid, sulfonic acid and hydrochloric acid. However, the response is 4000 times slower than the primary catalyzed reaction, usually requiring a higher ratio of alcohol/oil and the temperatures and pressure conditions are generally higher [43].
Alcohol-catalyst mixing
Before adding oil, the alcohol used in the production of Biodiesel must be mixed with a catalyst. The mixture is stirred until the catalyst is completely dissolved in the alcohol. It should be noted that alcohol should be anhydrous (anhydrous). Sodium hydroxide and potassium hydroxide are some of the most widely used alkaline catalysts. For production on an industrial scale, methoxide or sodium methoxide, or potassium methoxide is commercially available. The amount of NaOH/KOH used as a catalyst varies between 0.5% and 1.0%, depending on FFA’s raw material content [30]. When used as a catalyst, the amount of sodium methoxide or potassium methoxide is usually less than 0.5%. Potassium hydroxide is more suitable for the production of ethyl ester biodiesel. Both bases can be used for methyl esters.
Reaction time and temperature
The reaction speed and time required to achieve completion vary significantly with temperature and pressure. The reaction rate increases with increasing temperature, but in order to reduce heating costs, the reaction should be performed at approximately room temperature and atmospheric pressure [42]. The separation of reaction products is done by decantation: the mixture of Fatty Acid Methyl Esters (FAMEs) is separated from glycerin, forming two phases because they have different densities. After stoppin the stirring, the two stages immediately began to form. Due to their various chemical affinities, most of the catalyst and excess alcohols will be concentrated in the lower phase (glycerol). In contrast, most of the monoglycerides, diglycerides and triglycerides will aggregate in the upper phase (FAMEs). Once the mesosphere is clearly defined and complete, these two phases can be physically separated.
Cost of biodiesel
The cost of Biodiesel is approximately 1.5% to 2% higher than conventional diesel due to the consumption of quality food edible oil. Therefore, the cost of raw materials is a significant contributor (60-80%) to biodiesel production costs [42]. If edible oil is replaced by inedible oil sources, such as jatropha, Karanja, Mahua, Linseed, Rubber seed, Neem, cottonseed, Soapnut oil, etc. Biodiesel production will be cheaper.
Present Research Project Hypothesis
In developing countries like Pakistan, compared to bio-origin fuels (biofuels), such as petroleum diesel, Biodiesel has become more important. Due to the stability, portability, agility and ecofriendliness of vegetable oils, it has become increasingly attractive to alter plant oil for Biodiesel; however, in contemporary ages, the practice of this vegetable oil for biofuel manufacture has become a significant problem since they contend directly with foodstuff. That is why it is unbearable to defend and prove the usage of edible oil in biofuel making. In this regard, this research project is limited to the investigation of inedible oil plant assets that have more and more potential quantitative and qualitative information about biodiesel production and composition/characterization. This is a small step towards green energy, but overcoming sustainable energy crises is the key to moving towards green energy.
Biodiesel in Pakistan
Due to the shortage of Petro diesel fuel and rising prices, India’s awareness of Biodiesel has recently increased. Its production has embarked on a large number of activities and projects, most notably to reduce the enormous costs involved in importing petroleum fuels and to address and solve the problem of Petro diesel shortages that are expected to occur within a few years from now. In addition, the process of producing biofuel from inedible plant oil will improve the rural economy and provide a clean, decaying and safe environment. The forecast of biodiesel demand in India and the corresponding area required for Jatropha planting to achieve the goal of blending/ mixing biodiesel and Petro diesel.
Selection Process for Biodiesel Production
Inexpensive, non-food crops, fast-growing and early fruiting, high oil content, non-toxic and biodegradable varieties are often preferred as biofuel sources. Under Indian conditions, these nonedible plant species can be grown in large quantities in the desert. They can be subdivided into fuel cells fraction, which can be considered for biodiesel production. Some well-known, Other nonedible oil-producing plants including Azadirachta indica (Neem), Boswellia ovalifololata, Calophyllum inophyllum (Nagchampa), Calotropis gigantia (Aak), Euphorbia tirucalli (Sher), Hevea brasilensis (Rubber), Jatropha curcas (Physic nut) and Pongamia pinnata (Karanj) [44].
Preference of Non-Edible Oil Feedstock for Biodiesel Production
In developing countries such as Pakistan, Biodiesel, bioethanol and biogas are becoming increasingly more important than Petro diesel [45]. However, a significant challenge to the broad adoption of biomass energy to sustain its sustainability is the current edible oil feedstock used for its production. Globally, edible raw materials or consumer goods are facing the challenge of food competition. Because of this obstacle, biodiesel researchers, policymakers and industrialists are looking for new inedible oilseed resources to overcome current food challenges. In the above context, other promising non-edible oil plants species studied in the literature include physic nuts [46], pongame [9,47-49], Rapeseed [50], Jatropha [51,52]; Castor Plant [53], Okra [54], Chinese tallow [53], Neem [48-53], Soapnut [53], yellow oleander [53], rubber seeds [53], hemp plant [9], etc. It is used as an important source of biodiesel production.
Biodiesel Technology
Since the advent of diesel engines, people have realized that they are more likely to use oil as fuel. In the late 1800s, Rudolf Diesel successfully demonstrated his invention, namely diesel engines, by using peanut oil. For the first time, they determined the possibility of using plant-derived oil for the machine. However, due to their high viscosity, direct use of these oils instead of conventional fuels will encounter operational/performance issues. Later, due to technological progress and advances, three key technologies have been used to produce Biodiesel from oil.
Thermal cracking
Thermal cracking/pyrolysis is defined as converting one substance to another by heating in the absence of air or oxygen at a temperature of 450 to 850 °C, or with the help of the Lewis acid catalyst. The Lewis acid catalyst used in this process includes zeolite (M2/nO.A1203.x5102.yH2o), clay montmorillonite, Aluminum Chloride (AICL3), Aluminum Bromide (AlBr3), Iron Chloride (FeCi.z) and Ferrous Bromide (FeBr1). However, removing oxygen during heat treatment also removes the environmental/natural benefits of using oxygenated fuels [55]. In addition, these fuels are more like gasoline than diesel
Microemulsion
A microemulsion is technically defined as a stable dispersion of one liquid phase into another liquid phase, with a droplet diameter of approximately 100nm or less. Micro emulsification methods have been studied to produce Biodiesel to increase the viscosity of vegetable oils by mixing with simple alcohol, namely methanol or ethanol [56,57]. However, it has been reported that, in the long run, the fuel produced by this process has obvious sticking of the injector’s needle exposure, carbon deposit, insufficient heat combustion, and increased lubricating viscosity of oil [57].
Transesterification
The transesterification reaction is a reversible reaction process that involves the conversion of esters into different esters. To make Biodiesel, a transesterification reaction was performed to reduce the viscosity of vegetable oil. Specifically, triglyceride molecules react with low molecular weight alcohols such as methanol or ethanol to produce monoalkyl esters and by-product glycerol, which are used in the pharmaceutical industry. Chemically, the fundamental dogma of biodiesel synthesis and its composition (transesterification step) is shown in Figure 3. But today, before a continuous base-catalyzed transesterification reaction, two-step acid pretreatment is the best common technique of processing crude oil into biofuel [58-60] used a two-step esterification reaction of sulfonic acid-catalyzed to lessen the acid gratified of a high free fatty acid feedstock supply of less than 1wtp/ci. The twostep catalytic process has recently proven to be a cost-effective and efficient way to produce Biodiesel from waste edible oils by an acid content of 75.9mg KOH/g [43]. Simple liquid acid catalysts, such as Sulfuric Acid (H2SO4), Hydrochloric Acid (HCl) and other catalysts, are all effective catalysts for direct esterification. In the commercial process of trading systems, sulfuric acid was the catalyst of choice for organic synthesis in the esterification reaction. Use 1.7% by weight% H2SO4, had a high catalytic activity was obtained and the acid conversion rate was 90% [61].
Figure 3:Three-step reaction of transesterification (cited from e-education.edu.psu).

Among the above technologies, the transesterification reaction is considered to be one of the best feasible biodiesel production methods. Currently, most biodiesel production is carried out using alkaline-catalyzed transesterification reactions, as it can be carried out under mild conditions to achieve significant conversion with minimal side reaction in the reaction time. However, normal biodiesel production is affected by free fatty acids in the feedstock. The formation of free fatty acids in the presence of a fundamental homogenous catalyst will produce soap, create serious product separation problems and ultimately hindering/prevent catalytic activity. As a result, the process requires highly refined oil. Alternatively, the raw material should take precautionary measures to reduce acid concentration under the optimal threshold, which is 1wt.% of free fatty acids [62].
Catalyst’s Kinetics
Typically, biomass oil is trans esterified through two types of catalysts, i.e., homogenous and heterogeneous catalysts. To achieve cost-effectiveness and high biodiesel yields, potential catalysts must be used. However, in the preparation of Biodiesel, catalytic activity plays an important role. In this review, we briefly review the various forms of catalysts.
Homogeneous catalysts
Two types of homogenous catalysts are used to produce Biodiesel, alkaline homogeneous catalysts and homogeneous acid catalysts. Alkaline homogeneous catalysts are suitable for oilcontaining low free fatty acid contents because the high free fatty acid content that leads to saponification/can lead to soap formation and cause difficulties in glycerol separation. At the same time, the homogeneous acid catalyst is best suited for edible oils with high free fatty acid content.
Base homogeneous catalysts
The corresponding primary homogenous catalyst contains Sodium Hydroxide (NaOH), Sodium Methoxide (CH3NaO), Potassium Hydroxide (KOH) and Potassium Methoxide (CH3OK) [30,63-66]. These catalysts are easy to use and are economical because the process is carried out with low temperatures, short time, and atmospheric pressure. In parallel, the conversion rate is very high without intermediate steps
Acidic homogeneous catalyst
The acidic homogeneous catalysts are Sulfuric Acid (H2SO4), Phosphoric Acid (H3PO4) and Hydrochloric Acid (HCl), or organic sulfonic acid [30,67,68]; compared with essential homogenous catalysts, these catalysts require more time and higher temperatures to convert oil into Biodiesel ultimately. Acid-catalyzed transesterification methods are not popular in a commercial application. The fact is that acid-catalyzed reactions are 4000 times slower than those of based catalyzed [69] reactions. More recently, it has been shown how acid-catalyzed Biodiesel can compete economically with bases catalyzed by virgin oil [23,70].
Heterogeneous catalysts
The heterogeneous catalysts contain basic and acids solids, such as Magnesium Oxide (MgO), Calcium Oxide (CaO), aluminummagnesium hydrotalcite, Lithium Oxide (Li2O), Zinc Oxide (ZnO), Calcium Carbonate (CaCO3) and sulfated Zirconium oxide. Heterogeneous catalysts often require high reaction temperatures, high alcohol oil to molar ratio and longer reaction time. All of these concerns make biodiesel synthesis difficult and expensive [36,70- 75]. It is well known and widely documented that homogenous primary catalysts are becoming increasingly important on the industrial scale because they are more active and functional. Compared with heterogeneous catalysts, it takes less time and cost to convert oil into Biodiesel [21,30,63-66] and [30] observed a similar result effect, i.e., after 60 minutes, the molar ratio of 1:6 of the alkaline homogeneous catalyst NaOH to KOH was 1% (w/w), when the maximum activity and excellent work is accomplished.
Biodiesel Compatibility Standards
During the transesterification reaction, an intermediate molecule of monoglyceride, a diglyceride of glycerol, is formed, a tiny amount of which remains in the final product. In addition, there is a variety of unreacted triacylglycerol molecules, partial glycerol, unseparated glycerol, free fatty acids and residues! Alcohol and residual catalyst can contaminate the final product of Biodiesel. These pollutants can cause serious problems, such as engine deposit failure and fuel degradation, and leakage. For this purpose, the international principles ASTM D-6751 and EN 14214, Australian provisional biofuel standard, Brazilian provisional biofuel standard and South African biodiesel prov. Standard is designed to measure and quantify the limits of pollutants in Biodiesel [7,62,76-78]. In the United States, Biodiesel is regulated by ASTM D-6751, and in Europe, standard EN 14214 is used for biodiesel regulation [7]. In accordance with the rules and regulations of these standards set by law, certain parameters are specified in all physical and chemical tests. The specification limits of various biodiesel fuel tests are a fact, i.e., their irregularity can cause many serious problems in engine performance, efficiency, and life [7,62,79,80]. According to established international standards, the restrictions on free pollutants and total glycerol are restricted on individual and direct pollution. For example, free glycerin and total glycerin are the limits used to determine the content of glycerol and acylglycerol content. Flashpoint value is used to limit the residual alcohol, the free fatty acid content is used to reduce the amount of acid and the amount of ash is used to limit the remaining catalyst present in Biodiesel [62,79,80]; some methods are used to analyze Biodiesel to determine the location of contaminants in the final product of Biodiesel, such as water content, sulfur and phosphorus [80]. Special care and attention are required to meet the requirements of biodiesel standards qualitatively. It is not necessary to measure the individual compounds in Biodiesel but to measure and characterize each biodiesel sample’s chemical class. The fatty acid methyl ester, for example, the position of fatty acid methyl ester is saturated or unsaturated and its derivatives. The following analytical methods, such as GCMS chromatography, FT-IR, and 1H & 13C NMR spectroscopy, are considered to be the most reliable methods of biodiesel qualitative testing [7,81]. Therefore, in this case, determining the physical and chemical testing of Biodiesel and its blends is essential for successful marketing.
Biodiesel Characterization/Instrumentation
A suitable biodiesel analysis method will be able to perform low and reliable values to measure all traces of impurities in the Biodiesel and its mixture and to monitor the ongoing reaction. Individually, there is currently no analytical method that can meet the extreme need and requirements. Therefore, flexibility is necessary when selecting biodiesel analytical methods and their blends or monitoring the ongoing transesterification reaction. Because of technological advances such as Gas Chromatography- Mass Spectrometry (GCMS), chromatography Fourier Transforms Infrared Spectroscopy (FTIR) and Liquid Chromatography-Mass Spectrometry (LCMS) [7,81], the above categories often overlap in organic analytical chemistry. A major obstacle to high-cost analytical and investment technology, especially with regard to skills and technology development. It is very important to note that the confirmation and satisfaction levels of biodiesel standards do not need to be quantified by a single compound in Biodiesel but can measure different compounds that are present simultaneously. For example, studying the total amount of glycerol is not important. Based on monoacylglycerol glycerol, the detection of diglycerol and triacylglycerol is similar. A study of total glycerol is not essential. In this case, which type of acylglycerol is the source of glycerol sterns arising from, in this context, the most reliable and simple biodiesel analytical method for gas chromatography, Fourier transfer infrared spectroscopy, atomic absorption spectroscopy, and proton and carbon nuclear magnetic resonance spectroscopy [7,62,79].
Gas Chromatography (GC)
In general, among gas chromatography, high-performance liquid chromatography, gel-permission chromatography and thin-layer chromatography techniques, GC chromatography is considered to be the most beneficial method for measuring fatty acid methyl esters because of their high accuracy and precision. Freedman [30] used GC chromatography for the first time to determine the amount and conc. Of various fatty acid methyl esters and mono, di, total and free amount of glycerol in Biodiesel. In one of the first research works, [30] used a 1.8m short-fused silica capillary column for biodiesel research, while [82] quoted its typically fused silica capillary. The column was used for the quantitative analysis of rapeseed oil biodiesel. In many biodiesel research activities, GC chromatography has been used to determine specific compounds in fatty acid methyl esters. The determination of other contaminants (such as ethanol, methanol and free glycerol) only requires changes in column temperature and internal standard [62,83,84]. The column temperature system depends on the combination to be [62,85]. In this work, the column oven process system for the methyl esters analysis started at 150 °C for 1 minute, then at 20- 225 °C for 5 minutes and 5-250 °C for 2 minutes [86].
Nuclear Magnetic Resonance (NMR) spectroscopy
First, 1H NMR of [87] has been used to measure the conversion of triglycerides to fatty acid methyl esters after transesterification. In their research work, they used methylene protons close to half of the methyl ester in triglycerides and protons in the alcohol component of the methyl ester product to monitor biodiesel production yield (equation 1) [7].
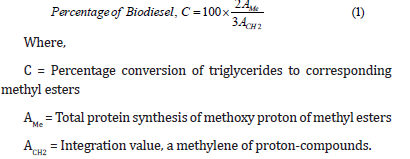
In the above formula, factors 2 and 3 are taken from the fact that methyl carbon has two protons, and alcohol carbon has three protons attached. Based on the above background, [9] used LH NMR to determine the degree of biodiesel conversion of vegetable hemp. Similarly, [88] added methanol to the rapeseed oil and used 13C NMR to test the kinetics of the transesterification response. [9] Applied carbon Nuclear Magnetic Resonance (13CNMR) to hemp oil biodiesel. In hemp oil biodiesel, the peak feature appeared at 174.2ppm, while the peak occurred at 131.92 and 127.10ppm, indicating that the peaks of methyl and methylene carbons that were not smeared with sesame oil came from the long carbon range of 14.0ppm (20.5-34.0ppm) [89].
Fourier Transform Infra-Red (FT-IR) spectroscopy
Fourier Transform Infrared Spectroscopy (FT-IR) has been cast-off to categorize and classify several efficient sets and bands associated with various stretching and bending vibrations in triacylglycerols and methyl esters [7]. According to [90], the position of the carbonyl group in the FTIR spectrum is sensitive to the effect of a substitute and molecular structure. In the FT-IR spectrum, the ester produced two strong absorption bands. These absorption bands may be caused by carbonyl groups (vC=O). The absorbent band appears from approximately 1750-1730cm-1: methylene, stretching vibration of methylene groups, methyne and olefin groups appear at 2980-2950, 2950-2850 and 3050-3000cm- 1, respectively and the bending vibrations of these groups are at 1475-1350, 1350-1150 and 722cm-1, respectively appeared [91]. It has been clearly stated that the use of analytical technology in bulk biodiesel is reasonable and reliable.
Inductively Coupled Plasma Spectroscopy (ICP-OES) and Elemental Analyzer (EA)
It is very important to specify the concentration of Sodium (Na), Potassium (K), Magnesium (Mg) and Calcium (Ca) in biodiesel, as their presence can cause solid deposits in the engine system. However, the utilization rate of use of these metals in biodiesel is very low. According to EN 14214 standard, the corresponding EN 1408 and EN 14538 standardized test methods are analyzed for concentrations of potassium, magnesium, sodium and calcium [7]; www.CrimsonEnergy.com; [78]; www.crmstandard.com].
The primary and most considerable advantage of metals in biodiesel and its blends is that they are very stable at high temperatures. This means that they will show lower bleed, improve chemical performance, and have a longer working life, making them less expensive options. They can also be heated to higher temperatures to allow the use of nonvolatile materials and improve engine life [7].
Diversity of Oil Seed Resources for Biodiesel Production in Pakistan
Geographically, Pakistani flora is vibrant and diverse. According to Hassan H et al. [92], Pakistan is naturally a center for flowering diversity. In Pakistan, there is no doubt that soil quality is very good for producing a wide variety of oil-producing plants. Pakistan is a large country with a variety of climates, i.e., topography and ecological regions. Accor. The flora used in each aspect is quite different depending on the variety and location of other geographical areas and regions. According to studied literature by Hassan H et al. [92], there are 6,000 species of flowering plants in Pakistan. Geographically, Pakistan is divided into four central phytogeographic regions, namely: Irano-Turanian regions, followed by Sino-Himalayas, Saharo-sindian regions and Indian regions (46%, 10%, 9%. 5% and 4.5%) respectively [93,94]. In this case, it is clear that due to the diversity of ecological features, Pakistan is geographically fortunate and lucky to have hundreds of oilproducing seed plants that can grow in some way throughout this seasonal period. Its planting/cultivation depends on the yield of the crop and its market value. Among them are other well-known oilproducing plants that can be used for biodiesel production, such as sunflower (Helianthus annus L.), linseed (Linum usitatissimum L.), Pongamia pinnata L., castor bean plant (Ricinus). Oat plant (Avena sativa), Carthamus plant (Carthamus oxyacantha L.), safflower (Carthamus tinctorius), soybean (Glycine) max), sesame plant (Sesamum indicum), milk thistle (Silybum marianum L.) Gaert, Aamla (Phyllanthus emblica L.), rocket seed (Eruca sativa L.), Cotton (Gossypium hirsutum L.), olive tree (Yea ferruginea), hemp plant (Cannabis sativa L.), Neem plant (Azadirachta indica Mrs Juss), Soapnut (Sapindus mukorossi) and palm oil [9,95-98]. In a particular season, its maximum yield depends on the climatic/ weather conditions.
Pakistan is a developing country, and it has long been required to provide cheap energy. At the same time, energy costs are also increasing and reaching a peak/higher. To further provide energy, Pakistan uses a variety of technologies, but the most dedicated to hybrid power, fossil fuel/mineral power and nuclear power. In this regard, biomass energy has aroused much interest because it is produced from renewable and sustainable biomass sources, and the biggest problem for global green energy has been low-cost biofuel resources [94]. Typically, oil extracted from the plant is converted to methanol by means of a catalyst for biodiesel generation. In general, methanol is used to produce biodiesel in the global market because of its better performance. In the above case, the source of methanol is coal. Pakistan’s coal resources are estimated at 180 billion tons, which is the world’s largest 5th Position (Ministry of Petroleum and Natural Resources, Government of Pakistan, 2011). In Pakistan, there is enough Sodium Chloride (NaCl) and its products can be further increased. Large amounts of sodium chloride can be used as crude factual for sodium hydroxide [Sitara Chemical Industries Pvt. Ltd and Inter-Vend Pvt. Ltd.]. Pakistan, known for its agriculture, must recognize/realize the importance of fertile soil and cooking oil in direct competition with food and safety. In addition, better non-edible oil plants can be easily planted in the soil of the burnt land.
For example, Jatropha plants can be grown in Pakistan, especially in salty and dry soils; drought and high temperatures can resist/withstand the Jatropha plant [India Jatropha Biodiesel Promotion Center of Excellence]. According to the report, the Jatropha plant can produce up to 2,240kg (2 tons) of biodiesel per hectare per year. In this way, if Pakistan uses all the reserves of burn land to grow Jatropha, we can get 56 million tons (6272’6kg) of biodiesel annually. In comparison, the current demand for Petro diesel is 8.5 million tons (504kg) [Department of petroleum and Natural Resources, Government of Pakistan]. In short, it is clear that in Pakistan, waste burn land can be used to grow non-edible oil plant varieties and a large number of raw materials can be used to produce biodiesel. In this regard, this research is possible in China and Pakistan and its prospects are very bright from a current and future perspective. Currently, in Pakistan, the ongoing energy demand is growing in this way. The Government of Pakistan attaches great importance to addressing the energy needs of the various sectors of the alternative energy sector.
Study/Research objectives
The restricted availability of raw material resources and the high prices of biodiesel production are a significant challenge for the industry. The expenses of the raw materials and the processing of the biodiesel are the two other variables that are relevant to the overall cost of the biodiesel synthesis process. The production of biodiesel currently entails or incorporates the utilization of a wide variety of edible oils and catalysts that are used in the biodiesel business; the specifics of which are determined by the processing costs. To create biodiesel commercially feasible and competitive with petroleum diesel, a constant procedure must be done to replace edible oil resources with non-edible raw materials and the quality of the product must be sacrificed in the process. In the end, the investment in a fundamental alkali catalyst optimization programmed system can cut down on the number of separation processes, which ultimately results in more in this respect.
Conclusion
Biodiesel is an excellent diesel engine alternative fuel due to its renewable and eco-friendly characteristics. Although various techniques exist for the production of biodiesel, transesterification of vegetable oil and lipids is currently the most prevalent. While researchers primarily concentrate on the cost reduction of biodiesel produced with non-edible oils, the utilization of consumable oils in this process has significantly advanced the field. As a long-term supplement, governments should maximize the utilization of these biodiesel resources and assure diesel security on an international level. Biodiesel exhibits comparable combustion characteristics to diesel and its engine power output has been determined to be equivalent to that of diesel. Additionally, the utilization of biodiesel in diesel engines leads to a significant decrease in engine emissions. As a result of this evaluation, we have reached the conclusion that biodiesel is a superior renewable fuel substitute for diesel.
Conflicts of Interest
I (Dr. Inam Ullah Khan) confirm as an author and declare that there are no known conflicts of interest associated with this publication.
References
- Berrios M, Skelton RL (2008) Comparison of purification methods for biodiesel. Chemical Engineering Journal 144(3): 459-465.
- Khan IU, Long H, Yu Y (2023) Potential and comparative studies of six non-edible seed oil feedstocks for biodiesel production. Int J Green Energy 1-22.
- Leung DY, Wu X, Leung MK (2010) A review on biodiesel production using catalyzed transesterification. Applied Energy 87(4): 1083-1095.
- Khan IU, Wei H, Li Y, Elmanshawy A, Li M, et al. (2023) Thevetia peruviana seed oil transesterification for biodiesel production: An optimization study. J Biomed Res Environ Sci 4(1): 064-076.
- Atadashi IM, Aroua M Kaziz AA (2010) High-quality biodiesel and its diesel engine application: A review. Renewable and Sustainable Energy Reviews 14(7): 1999-2008.
- Khan IU, Haleem A, Khan AS (2022) Non-edible plant seeds of Acacia farnesiana as a new and effective source for biofuel production. RSC Adv 12(33): 21223-21234.
- Knothe G, Steidley KR (2004) Lubricity of components of biodiesel and petro diesel. The origin of biodiesel lubricity. Energy and Fuels 19(3): 1192-1200.
- Khan IU, Haleem A (2022) A seed of Albizzia julibrissin wild plant as an efficient source for biodiesel production. Biomass Bioenerg 158: 106381.
- Khan IU, Chen H, Yan Z, Chen J (2021) Extraction and quality evaluation of biodiesel from six familiar non-edible plants seeds Sources. Processes 9(5): 840.
- Shah S, Sharma A, Guptha MN (2004) Extraction of oil from Jatropha curcas seed kernels by enzyme assisted three-phase partitioning. Industrial Crops and Products 20(3): 275-279.
- Khan IU, Yan Z, Chen J (2019) Optimization, transesterification and analytical study of Rhus typhina non-edible seed oil as biodiesel production. Energies 12(22): 1-21.
- Khan IU, Yan Z, Chen J (2020) Production and characterization of biodiesel derived from a novel source koelreuteria paniculata seed oil. Energies 13(4): 1-15.
- (2010) Anonymous, agricultural statistics of Pakistan 2008-2009. Government of Pakistan, Ministry of Food and Agriculture (Economic Wing), Islamabad, Pakistan.
- Khan IU, Yan Z, Chen J, Chen H, Shah SA (2021) Albizzia julibrissin: A non-edible seed oil source for biodiesel production. Int J Sci Eng Res 12(1): 327-341.
- Khan IU, Yan Z, Chen J, Chen H, Shah SA (2021) Acacia farnesiana seed oil: A promising source of biodiesel production. Int. J Sci Eng Res 12(1): 277-295.
- Cheng SF, Choo YM, Ma AN, Chuah CH (2004) Kinetics study on transesterification of palm oil. Journal of OiI Palm Research 16(2): 19-29.
- Ahmad M, Teong LK, Sultana S, Khan IU, Zuhairi AA, et al. (2015) Optimization of biodiesel production from Carthamus tinctorius Cv. Thori 78: A novel cultivar of safflower crop. Int J Green Energy 12(5): 447-452.
- Khan IU, Ahmad M, Khan AU (2015) Chemistry and elemental analysis of Carthamus tincturoius Thori-78. J Am Acad Res 3(5): 81-90.
- Wiebe R, Nowakowska J (1949) The technical literature of agricultural motor fuels: Physical and chemical properties, engine performance, economics, patents and books. Agecon Search 276.
- Khan IU, Shah SA (2021) Optimization and characterization of novel & non-edible seed oil sources for biodiesel production. Intech Open 39-56.
- Khan IU (2023) Biomass & bioenergy; case studies bioresources, chemical and biological processes, biomass products for sustainable, renewable energy and materials. Chem Eng Process Tech 8(2): 1080.
- Fadhil AB, Dheyab MM, Abdul-Qader AQ (2012) Purification of biodiesel using activated carbons produced from spent tea waste. Journal of the Association of Arab Universities for Basic and Applied Sciences 11(1): 45-49.
- Zhang J, Jiang L (2008) Acid-catalyzed esterification of Zanthoxylum bungeanum seed oil with high free fatty acid for biodiesel production. Bioresource Technology 99(18): 8995-8998.
- Hanh HD, Okitsu K, Nishimura R, Maeda Y (2009) Biodiesel production through transesterification of triolein with various alcohols in an ultrasonic field. Renewable Energy 34(3): 766-768.
- Joshi H, Moser BR, Toler J, Walker T (2010) Preparation and fuel properties of mixtures of soybean oil methyl and ethyl esters. Biomass and Bioenergy 34(1): 14-20.
- Nimcevic D, Puntigam R, Wörgetter M, Gapes JR (2000) Preparation of rapeseed oil esters of lower aliphatic alcohols. Journal of the American Oil Chemists' Society 77(3): 275-280.
- Sanli H, Canakci M (2008) Effects of different alcohol and catalyst usage on biodiesel production from different vegetable oils. Energy & Fuels 22(4): 2713-2719.
- Warabi Y, Kusdiana D, Saka S (2004) Biodiesel fuel from vegetable oil by various supercritical alcohols. Appl Biochem Biotechnol 113-116: 793-801.
- Marjanović AV, Stamenković OS, Todorović ZB, Lazić ML, Veljković VB (2010) Kinetics of the base-catalyzed sunflower oil ethanolysis. Fuel 89(3): 665-671.
- Freedman B, Fryde EH, Mounts TL (1984) Variables affecting the yields of fatty esters from transesterified vegetable oils. Journal of American Oil Chemical Society 61: 1638-1643.
- Ali LH, Fadhil AB (2013) Biodiesel production from spent frying oil of fish via alkali-catalyzed transesterification. Energy Sources Part(A) Recovery Utilization and Environmental Effects 35(6): 564-573.
- Berchmans HJ, Morishita K, Takarada T (2013) Kinetic study of hydroxide catalyzed methanolysis of Jatropha curcas-waste food oil mixture for biodiesel production. Fuel 104: 46-52.
- Mollenhauer T, Klemm W, Lauterbach M, Ondruschka B, Haupt J (2010) Process engineering study of the homogenously catalyzed biodiesel synthesis in a bubble column reactor. Industrial & Engineering Chemistry Research 49(24): 12390-12398.
- Rashid U, Anwar F, Moser BR, Ashraf S (2008) Production of sunflower oil methyl esters by optimized alkali-catalyzed methanolysis. Biomass and Bioenergy 32(12): 1202-1205.
- Lee JS, Saka S (2010) Biodiesel production by heterogeneous catalysts and supercritical technologies. Bioresource Technology 101(19): 7191-7200.
- Liu X, He H, Wang Y, Zhu S, Piao X (2007) Transesterification of soybean oil to biodiesel using CaO as a solid base catalyst. Fuel 87(2): 216-221.
- Semwal S, Arora AK, Badoni RP, Tuli DK (2011) Biodiesel production using heterogeneous catalysts. Bioresource Technology 102(3): 2151-2161.
- Gamba M, Lapis AA, Dupont J (2008) Supported ionic liquid enzymatic catalysis for the production of biodiesel. Advanced Synthesis and Catalysis 350(1): 160-164.
- Iso M, Chen B, Eguchi M, Kudo T, Shrestha S (2001) Production of biodiesel fuel from triglycerides and alcohol using immobilized lipase. Journal of Molecular Catalysis B: Enzymatic 16(1): 53-58.
- Nielsen PM, Brask J, Fjerbaek L (2008) Enzymatic biodiesel production: Technical and economic considerations. European Journal of Lipid Science and Technology 110(8): 692-700.
- Sotoft LF, Rong BG, Christensen KV, Norddahl B (2010) Process simulation and economical evaluation of enzymatic biodiesel production plant. Bioresource Technology 101(14): 5266-5274.
- Bart JC, Palmeri N, Cavallaro S (2010) Biodiesel science and technology: From soil to oil. (1st edn) Wood head Publishing Ltd, UK, pp. 663-664.
- Sarivastava A, Prasad R (2000) Triglycerides-based diesel fuels. Renewable and Sustainable Energy Review 4(2): 111-133.
- Sudarsan KG, Anupama PM (2006) The relevance of biofuels. Current Science 92(6): 748-749.
- Barnwal BK, Sharma NP (2005) Prospectus of biodiesel production from vegetable oil in India. Renewable and Sustainable Energy Reviews 9(4): 363-378.
- Tiwari AK, Kumar A, Raheman H (2007) Biodiesel production from Jatropha oil (Jatropha curcas) with high free fatty acids: An optimized process. Biomass and Bioenergy 31(8): 569-575.
- Vivek, Gupta AK (2003) Biodiesel production from Karanja oil. J Sci Indus Res 63: 39-47.
- Rao NV, Krishna MV, Murthy PV (2013) Comparative studies on exhaust emissions and combustion characteristics of a ceramic coated diesel engine with tobacco seed oil-based biodiesel. International Journal of Advanced Scientific & Technical Research 3(5): 334-349.
- Paul PF, Wise WS (1971) The principle of gas extraction. Mills and Boon, London 18(3): 670-671.
- Qiu F, Li Y, Yang AD, Li X, Sun P (2011) Biodiesel production from mixed soybean oil and rapeseed oil. Applied Energy 88(6): 2050-2055.
- Hanny JB, Shizuko H (2008) Biodiesel production from crude Jatropha curcas seed oil with a high content of free fatty acids. Bioresource Technology 99(6): 1716-1721.
- Pradip J, Dinakran PM, Srikanth B (2010) Radiation doses during chest examinations using dose modulation techniques in multislice CT scanner. The Indian Journal of Radiology & Imaging 20(2): 154-157.
- Atabani AE, Silitonga AS, Badruddina IA, Mahliaa TM, Masjukia HH, et al. (2012) A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renewable and Sustainable Energy Reviews 16(4): 2070-2093.
- Farooq A, Rashid U, Ashraf M, Nadeem M (2009) Okra (Hibiscus esculantus) seed oil for biodiesel production. Applied energy 87(3): 799-785.
- Issariyakul T, Kulkarni MG, Meher LC, Dalai AK, Bakhshi NN (2008) Biodiesel production from mixtures of canola oil used cooking oil. Chemical Engineering Journal 140(1-3): 77-85.
- Mano S, Noomhorm A, Anal AK (2014) Influence of combined far‐infrared and superheated steam for cooking chicken meat patties. Journal of Food Process Engineering 37(5): 515-523.
- Vicente G, Martínez M, Aracil J (1998) Application of the factorial design of experiments and response surface methodology to optimize biodiesel production. Industrial Crops and Products 8(1): 29-35.
- Lapuerta M, Armas O, Fernandez JR (2008) Effect of biodiesel fuels on diesel engine emissions. Prog Energy Combust Sci 34(2): 198-223.
- Canakci M, Gerpen JV (2001) Biodiesel production from oils and fats with high free fatty acids. Transactions of American Society for Agricultural Engineers 44(6): 1429-1436.
- Zullaikah S, Chao CL, Ramjan VS, Yi HJ (2005) A two-step acid-catalyzed process for biodiesel production from rice bran oil. Bioresource Technology 96(17): 1889-1896.
- Khan AK (2002) Research into biodiesel kinetics & catalyst development. University of Queensland: Brisbane, Australia.
- Mittelbach M (1996) Diesel fuel derived from vegetable oils, VI: Specifications and quality control of biodiesel. Bioresource Technology 56(1): 7-11.
- Tanaka Y, Okabe A, Ando S (1981) Method for the preparation of a lower alkyl ester of fatty acids. US Patent 4: 303-590.
- Noureddini H, Zhu D (1997) Transesterification of soyabean oil. Journal of the American Oil Chemists' Society 74: 1457-1463.
- Mohamed MS, Bornscheuer UT (2003) Improvement in the lipase-catalyzed synthesis of fatty acid methyl esters from sunflower oil. Enzyme and Microbial Technology 33(1): 97-103.
- Evera T, Rajendran K, Saradha S (2009) Biodiesel production process optimization and characterization to assess the suitability of the product for varied environmental conditions. Renewable Energy 34(3): 762-765.
- Widyan MI, Shyoukh AO (2002) Experimental evaluation of the transesterification of waste palm oil into biodiesel. Bioresource Technology 85(3): 253-256.
- Zhang WF, He YL, Zhang MS, Yin Z, Chen Q (2000) Raman scattering study on anatase TiO2 Journal of Physics D: Applied Physics 33(8): 912.
- Zhang Y, Dubé MA, McLean DD, Kates M (2003) Biodiesel production from waste cooking oil: 2. Economic assessment and sensitivity analysis. Bioresource Technology 90(3): 229-240.
- Shimada Y, Watanabe Y, Sugihara A, Tominaga Y (2002) Enzymatic alcoholysis for biodiesel fuel production and application of reaction to oil processing. J Mol Catal B Enzyme 17(3-5): 133-142.
- Serio MD, Ledda M, Cozzolino M, Minutillo G, Tesser R, et al. (2006) Transesterification of soybean oil to biodiesel by using heterogeneous fundamental catalysts. Ind Eng Chem Res 45(9): 3009-3014.
- Kewashima A, Matsubara K, Honda K (2009) Acceleration of catalytic activity of calcium oxide for biodiesel production. Bioresource Technology 100(2): 696-700.
- Kulkarni MG, Dalai AK (2006) Waste cooking oils an economic source for biodiesel: A Review. Industrial and Engineering Chemistry Research 45(9): 2901-2913.
- Hartman L (1956) Methanolysis of triglyycerides. Journal of the American Oil Chemical Society 33: 129.
- Mittelbach M, Trathning B (1990) Kinetics of alkaline catalyzed methanolysis of sunflower oil. Fat Science Technology 92(4): 145-148.
- Ghulam Y, Muhammad B, Tariq MA, Syed M, Sibtain RN, et al. (2012) Quality of commercial high-speed diesel and its environmental impact. Journal of Petroleum Technology and Alternative Fuels 3(3): 29-35.
- Mittelbach M (1996) Diesel fuel derived from vegetable oils, VI: Specifications and biodiesel quality. Bioresource Technology 56(1): 7-11.
- Komers K, Skopal F, Stonkal R (1997) Determination of the neutralization number for biodiesel fuel production. Fett-Lipid 99(2): 52-54.
- Prince Alan, Tesar B (2004) Learning phonotactic distributions. Constraints in Phonological Acquisition 245-291.
- Cvengrosova Z, Cvengros J, Hronec M (1997) Rapeseed oil ethyl esters as alternative fuels and their quality control. Petroleum Coal 39: 36-40.
- Bondioli P, Mariani C, Fedeli E, Gomez AM, Veronese S (1992) Vegetable oil derivatives as diesel fuel substitutes: Analytical aspects. Note 3: Determination of methanol. Italian Journal of Fatty Substances 69(9): 467-469.
- Bondioli P, Mariani C, Lanzani A, Fedeli E (1992) Vegetable oil derivatives as diesel fuel Substitutes: Analytical aspects. Note 2: Determination of free glycerol. Riv Iral Sosrsan:e Grasse 69(1): 7-9.
- Mittelbach M (1993) Kinetics of alkaline catalyzed methanolysis. Chromatographia 37: 623.
- Pengmei L, Zhenhong Y, Lianhua L, Zhongming W, Wen L (2010) Biodiesel from different oil using fixed-bed and plug-flow reactors. Renewable Energy 35(1): 283-287.
- Gelbard GO, Bres RM, Vargas F, Vielfaure, Schuchardt UF (1995) 1H nuclear magnetic resonance determination of the yield of the transesterification of rapeseed oil with methanol. Journal of the American Oil Chemists' Society 72: 1239-1241.
- Dimmig T, Radig W, Knoll C, Dittmar T (1999) I3C-NMR spectroscopy to determine the conversion and reaction kinetics of the transesterification of triglycerides to methyl esters. Chemical Technology (Leipzig) 51(6): 326-329.
- Christie WW (2003) 13-Phenyltridec-9-enoic and 15-phenylpentadec-9-enoic acids in Arum maculatum seed oil. European Journal of Lipid Science Technology 105(12): 779-780.
- Pasto D, Johnson C, Miller M (1992) Experiments and techniques in organic chemistry. (1st edn), Prentice-Hall: Upper Saddle River, New Jersey, USA.
- Guillen MD, Cabo N (1997) Infrared spectroscopy in the study of edible oils and fats. Journal of the Science of Food and Agriculture 75(1): 1-11.
- Razvi, Salim, Saatcioglu M (1999) Confinement model for high-strength concrete. Journal of Structural Engineering 125(3): 281-289.
- Ali B, Shah GA, Traore B, Shah SA, Shah S, et al. (2019) Manure storage operations mitigate nutrient losses and their products can sustain soil fertility and enhance wheat productivity. Journal of Environmental Management 241: 468-478.
- Hassan H, Sima’an K, Way A (2007) Supertagged phrase-based statistical machine translation. In Proceedings of the ACL Prague Czech Republic 288-295.
- Demirbas A (2005) Biodiesel production from vegetable oils via catalytic and non-catalytic supercritical methanol transesterification methods. Progress Energy Combustion Science 31(5-6): 466-487.
- Chhetri AB, Tango MS, Budge SM, Watts KC, Islam MR (2008) Non-edible plant oils as new sources for biodiesel production. International Journal of Molecular Sciences 9(2): 169-180.
- Karmakar A, Karmakar S, Mukherjee S (2010) Properties of various plants and animal feedstocks for biodiesel production. Bioresource Technology 101(19): 7201-7210.
- Adun AN, Chukwulete A (2013) An assessment of the readership base for Nigerian blogs. Mgbakoigba: Journal of African Studies 2: 118-131.
- Schuchardta U, Serchelia R, Vargas RM (1998) Transesterification of vegetable oils: A review. Journal of Brazillian Chemical Society 9(3): 199-210.
- Pinto AC, Guarieiro LL, Rezende MJ, Ribeiro NM, Torres EA, et al. (2005) Biodiesel: An Overview. Journal of Brazilian Chemical Society 16(6b): 1313-1330.
© 2024 Inam Ullah Khan. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






