- Submissions

Full Text
Progress in Petrochemical Science
Recent Achievements in Microporous Metal-Organic Frameworks Based on Ionic Liquids/ Deep Eutectic Solvents for Efficient Removal of Toxic Gases and VOCs
Shima Rahmdel Delcheh1*, Alireza Pourvahabi Anbari2,3, Philippe M Heynderickx2,3 and Francis Verpoort4,5
1Department of Chemistry, Guilan University, Iran
2Department of Chemistry, Faculty of Science, Ghent University, Belgium
3Center for Environmental and Energy Research (CEER), Ghent University Global Campus, Republic of Korea
4State Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology, China
5National Research Tomsk Polytechnic University, Russia
*Corresponding author:Shima Rahmdel Delcheh, Department of Chemistry, Guilan University, Irana
Submission: September 19, 2023;Published: September 28, 2023

ISSN 2637-8035Volume5 Issue5
Abstract
In recent years, micro-porous metal-organic frameworks, often known as MOFs, have received a great deal of interest owing to the remarkable gas storage and separation characteristics that they possess. This literature review investigates the progressive concepts surrounding the integration of Metal-Organic Frameworks (MOFs) with Ionic Liquids (ILs) and Deep Eutectic Solvents (DESs) for the purpose of the effective capture of Volatile Organic Compounds (VOCs). This study provides a full overview of the achievements made in this area by discussing the synergistic effects and increased performance gained via the combination of MOFs with ILs and DESs. These effects were accomplished through the combination of MOFs with ILs and DESs. In addition, the difficulties and potential benefits of the future that are related to this forward-thinking attitude are discussed.
Keywords:Micro-porous Metal-Organic frameworks (MOFs); Integration; Ionic liquids (ILs); Deep eutectic solvents (DESs); Volatile organic compounds (VOCs)
Introduction
Capturing CO2 can be completed directly with the help of green plants absorbing the sunlight to convert CO2 and water into diverse hydrocarbons and oxygen through photosynthesis. This fundamental action occurs over a series of connected reactions which are catalyzed by special metallo-enzymes [1]. The main matter of the researchers that made a gradual concern is human-related climate change. In the current issues, the presence of carbon dioxide gas has a great effect [2]. In order to meet the daily demands of humans, fossil fuels which were a consequence of millions of years photosynthetic activity are considered as a convenient supplier. With the beginning of the industrial revolution, the consumption of fossil fuels has increased dramatically. Consequently, the concentration of the CO2 in the atmosphere increased consequently. During the recent years a huge amount of worries of the researchers and active environmental protection organizations made them concentrate on the universal energy deficiencies and environmental issues [3]. Human society tries to substitute renewable energies instead of fossil fuels and hereby reduce the CO2 emissions. One of the most favorable solutions is using solar technology which solves both the mentioned problems in CO2 emission via ascending the atmospheric CO2 levels and producing cherished compositions as chemical ingredients or fuels for energy consumption [4].
Finding a more stable technique such as photo-redox CO2 reduction catalysis can be an important challenge [5]. The capture, activation and successive reduction of the CO2, in a successful CO2 transformation, requests a super-fit catalyst, which has the ability to reduce through multi-electron transportation and prevention of thermodynamically huge energy consumption [6-7]. Hence, the creature and progress of techniques, in which carbon capture increases are in the socio-economic viewpoint. As an incredible exceptional gas storage source, Metal Organic Frameworks (MOFs) organize an important class in the mesoporous materials [8-10]. Because of the porous nature and tunability of the MOFs, they can store a large capacity of various gasses [11,12]. Also, it can be stated that moreover the potential of the CO2 conversion in MOFs is extraordinary, they depict a large amount of chemical and thermal stability. This feature of high tunability in MOFs makes them viable in the field of gas treatment [13-15] but the issue of selective separation as an important consideration must be defined which counts as an unanswered subject for MOFs in separation procedure. To overcome the aforementioned problem, mix matrix membranes and functionalization by setting the selective adsorption can be a proper solution [16-18]. Ionic liquids can be applied to metal surfaces for miscellaneous applications [19,20]. Besides the separation power and recycling ability they have a high potential in CO2 solubility [21] which leads to be a great candidate for gas handling procedures [22].
Likewise, the human is looking for eco-friendly absorbent materials showing high reactivity and sufficient surface region [23]. Despite all efforts in probing green and environmentally friendly techniques in the construction process of MOFs, it still needs more attention [24]. Remarkably, The quick progress in creating, designing and innovating new applications in the field of material science has stimulated the researchers to substitute old fashioned organic solvents for making the functional ingredients to achieve the moralities of Green Chemistry. Deep Eutectic Solvents (DESs) as a fast growing green solvent, via the features of biodegradability, nonvolatility, non-toxic and low cost, with the inherent characteristic of open metal sites in the organization of MOF could effectively support the pore size and also modify the pore surface and finally improve the adsorption selectivity of certain toxic gases which are detrimental to the environs [25-27]. So, these newcomer materials can also open up an undeniable consequence in catalytic and photo catalytic degradation of contaminants [28,29]. These green and eco-friendly solvents have been talented as great alternates for non-liquified solvents and room temperature ionic liquid solvents, as Abbott and his team had practical experiments on these moisture stable and unique quaternary ammonium salts that are functional in various applications the same as those previous MOFs [30-32]. In this research, the researcher will focus on the development and forecasts in the fabrication of novel and innovative MOFs using alternatives from green solvents (deep eutectic solvents), simulation of the studied systems with Molecular dynamic calculations and consider the data validation by Matlab software and finally apply the prepared MOFs for the adsorption and remediation of toxic gaseous (especially CO2 capturing) pollutants in the environment.
Literature Review
In recent years, there has been a substantial uptick in the investigation of micro-porous Metal-Organic Frameworks (MOFs), which are seen as potentially useful materials for the removal of harmful gases and Volatile Organic Compounds (VOCs). The discovery of crystalline coordination polymers in the 1990s led to the conception of Metal-Organic Frameworks (MOFs). These polymers are formed of metal ions or clusters that are coupled by organic ligands [33]. Since that time, a significant amount of research effort has been focused on the design and synthesis of MOFs with structures and characteristics that have been specifically tuned for a variety of applications, including the storage and separation of gases. A more recent development in this area is the combination of Metal-Organic Frameworks (MOFs) with Ionic Liquids (ILs) and Deep Eutectic Solvents (DESs) with the purpose of trapping hazardous gases and Volatile Organic Compounds (VOCs). ILs, which are organic salts with low melting points and have garnered a lot of interest owing to their distinctive features such as low volatility, thermal stability and tunability [34], are salts with low melting points and are classified as organic. On the other hand, DESs are eutectic mixtures that are generated by mixing a hydrogen bond donor (such as a quaternary ammonium salt) with a hydrogen bond acceptor (such as a metal halide) [35]. This process results in the formation of DESs. DESs have earned a reputation for being solvents that are safe for the environment and possess exceptional solubility and selectivity qualities.
When it comes to the removal of harmful gases and volatile organic compounds, a number of studies have shown that mixing MOFs with ILs and DESs may result in synergistic effects and improved performance. The addition of ILs and DESs to MOFs permits enhanced adsorption capabilities, selectivity and stability [36,37]. In addition, the addition of these functionalities provides MOFs with new functions. The interactions that take place between MOF frameworks and ILs/DESs play an essential part in the adsorption and separation processes, which ultimately results in a more effective removal of hazardous gases and volatile organic compounds. In general, the historical development of progressive views on micro-porous MOFs based on ILs and DESs for collecting dangerous gases and VOCs has opened the way for creative methods in gas storage and separation. These visions are based on the idea that micro-porous MOFs might be used to store and separate gases with very small pores. The combination of Inorganic Liquids (ILs) and Gaseous Electron Sources (DESs) with Metal- Organic Frameworks (MOFs) is a viable method to addressing the issues associated with environmental degradation and the need for environmentally responsible gas treatment technologies.
Construction and Synthesis of New MOFs
In order to produce MOFs in the presence of toxicants, researchers have, over the course of many years, used a technique called high-temperature annealing [38,39] that included a lengthy period of time [40]. Surfactants in which precipitate through rapid procedures play a vital function as agents to guide [41], which is a role that is essential for controlling the size and morphological tunability of MOFs-derived nano crystals. Nevertheless, throughout the course of the last several years, a variety of research concerning the independent competence of MOFs including DESs have been documented. The use of DESs as effective organic linkers will pave the way for the development of organisms composed of very permeable, tunable, and adaptable materials that thrive in favorable environmental circumstances [42-44].
Construction method
The synthesis method that is based on iono-thermal is the most advantageous and environmentally friendly technique to create these novel structures, consisting of zeolite, zeo-typemeso and macro porous material and metal-organic frameworks, which applied ionic liquid or DES as reaction media or structural matters [45,46]. These novel structures consist of zeolite, zeo-typemeso and macro porous material, and metal-organic frameworks. The approach that was just discussed may be used effectively throughout the manufacturing process of DES-MOF-based products. In this study, the researcher’s objective is to develop an efficient method for creating a reaction medium out of ionic liquids based on imidazolium. At first look, it seems as if the created MOF would have some restrictions when it comes to the adsorption and catalysis of gases. The next stage is to evaluate these composites in light of recently developed and cutting-edge structures. When it comes to imidazolium based ILs, the components in DESs serve more as supporting ligands than they do as the reaction medium. This results in the development of new MOFs that have more open holes that may be used as efficient materials [47].
The significance of the Advancement of DES-Based Metal–Organic Frameworks
MOFs based on imidazolium ILs for the capture of carbon dioxide
Ionic liquids based on imidazolium have a remarkable potential to trigger and then transform CO2 into fuels or other suitable compounds, as has been highlighted in a number of papers [48-50]. These liquids may be found in numerous forms. Although in control experiments, the construction of urea and its derivatives, for example, were near to zero in most cases once the imidazolium ILs were eliminated, and the presence of imidazolium ILs in separating CO2 seems to be accompanied by a high efficiency, ILs are not always environmentally friendly, inexpensive, or simple to prepare.
DES-based metal organic frameworks as a more suitable separator
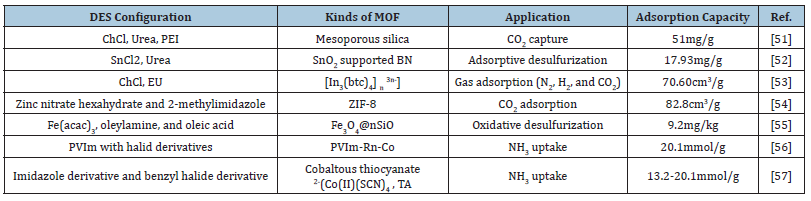
The preservation of a green and manageable environment should be the primary focus of all research efforts since it is of the utmost importance. As a result, the creation of MOFs based on DESs as a green medium has given an excellent environment for the adsorption and capture of harmful gases throughout the union. Some of the most significant developments pertaining to this matter have been brought to your attention in Table 1 [51-57].
Table 1:Development and application of MOFs based on DESs in toxic gases adsorption media. Affiliations: ChCl: Choline chloride; PEI: Polyethyleneimine; BN: Boron Nitride; DBT: Dibenzothiophene; btc: 1,3,5 benzenetricarboxylate; EU: Ethyleneurea; PVIm: Polyvinylimidazole.
Application of MOFs
The principal greenhouse gas contributing to climate change, Carbon Dioxide (CO2), may be captured and stored in Metal-Organic Frameworks (MOFs), which have shown significant promise as useful materials for this purpose. The enormous surface area, controllable pore size and chemical flexibility of MOFs make them promising candidates for CO2 collection. Incredibly high levels of adsorption capacity, selectivity and regeneration have all been established by MOFs in several research looking at their usage for CO2 collection. MOFs have found usage as adsorbents in postcombustion capture techniques for CO2. In order to efficiently separate and absorb CO2 from exhaust gas streams at power plants and industrial sites, MOFs can absorb CO2 selectively. MOFs like MOF-74 (CPO-27) and HKUST-1 (Cu-BTC) have been used successfully for CO2 collection [58,59]. These MOFs have significant CO2 adsorption capabilities and can be recycled for several uses, making them competitive candidates for industrial-scale CO2 collection. MOFs may also be used as adsorbents, in particular for gas separation and purification, in pre- combustion capture procedures. By manipulating their structures and functions, MOFs may selectively remove CO2 from gas mixtures, paving the way for the generation of ultra-pure hydrogen gas.
Mg-MOF-74 and UiO-66 are two Metal-Organic Frameworks (MOFs) that have shown promising CO2 adsorption selectivity in hydrogen purification [60,61]. Because of their strong attraction to carbon dioxide, they may effectively filter out contaminants and improve the cleanliness of the hydrogen gas that is produced. In addition, MOFs have shown promise for CO2 collection from unexpected sources, including capture from ambient air. By selectively adsorbing CO2 from air, MOFs open the door to atmospheric removal of this greenhouse gas. This strategy is gaining traction as a way to reduce carbon emissions and fight global warming. Amazing CO2 collection capabilities from ambient air have been exhibited by MOFs as MOF-177 and MOF-74 [62,63]. In conclusion, MOFs have been shown to be very flexible materials for CO2 collection. They may be optimized for post-combustion, precombustion, or ambient air collection because of their large surface area and selective adsorption characteristics. Researchers continue to make strides in the area of CO2 capture and contribute to the development of sustainable solutions for mitigating climate change by creating and synthesizing MOFs with optimum structures and characteristics.
Computational Methodology
DFT calculations
Calculations based on the Density Functional Theory (DFT) are one computational approach that might be used for this purpose. In order to anticipate and understand the electronic structure and behavior of materials, DFT is a strong quantum mechanical approach [64]. Micro-porous Metal-Organic Frameworks (MOFs), Ionic Liquids (ILs), Deep Eutectic Solvents (DESs) and hazardous gases or Volatile Organic Compounds (VOCs) may all be studied by DFT simulations in this context. The adsorption processes, binding energies and stability of the captured species inside the MOFs functionalized with ILs or DESs may be better understood with the use of DFT simulations of the molecular structures and energies of these systems. The electrical characteristics and reactivity of MOFs and their composite materials may also be investigated through DFT computations. Band structure, density of states, and frontier orbital analysis may all provide insight into the charge transfer processes and catalytic activity of the materials, which are essential for the removal of hazardous gases and volatile organic compounds [64-67].
To further improve gas capture efficiency, DFT computations may be used in the rational design and optimization of novel MOFs, ILs and DESs. Computational modeling may help guide the creation of innovative materials with enhanced adsorption capabilities, selectivity, and stability by carefully examining the impacts of various functional groups, ligands and solvents. For all the structures containing ILs and DESs, the geometries of the designed structures can be optimized using Becke’s three-parameter hybrid exchange functional and the Lee-Yang-Parr correlation functional (B3LYP) [65,66] with 6-311++G** basis set in Gaussian16 package [65]. CELLOPT protocol can be applied for optimizing the ILs and DESs single pairs connecting to the pore of ZIFs and to relax the composite structures. CELLOPT is used to expand the unitcell parameters for electron diffraction data of small-molecule crystals. The CELLPOT method will be applied to assist in relaxing the structures through regulating the original lattice parameters. Dispersion correction has an incredible effect on the estimation of properties of materials [66] and consequently so effectively in finding the structure of different ZIFs [67]. As a whole, using Density Functional Theory (DFT) calculations to investigate the mechanisms behind the capture of toxic gases and volatile organic compounds in micro-porous metal-organic frameworks based on ionic liquids and deep eutectic solvents can speed up the discovery and design of efficient gas capture materials.
GCMC simulations
Grand Canonical Monte Carlo (GCMC) simulations are another computing approach that might be used for this study. When it comes to porous materials like ionic liquid and deep eutectic solvent-functionalized Metal-Organic Frameworks (MOFs), GCMC simulations are an invaluable tool for learning about adsorption and gas separation processes. It is possible to calculate adsorption isotherms, selectivity, and diffusion characteristics using GCMC simulations since they include the random insertion and removal of gas molecules into a porous medium. Adsorption behavior of hazardous gases and Volatile Organic Compounds (VOCs) within the micro-porous MOFs functionalized with ILs or DESs may be studied using GCMC simulations in the context of this issue. This helps provide light on how various solvents perform in terms of adsorption capacity, selectivity and gas capture. Adsorption enthalpy and entropy are two thermodynamic variables of the gas capture process that may be investigated using GCMC simulations. These characteristics may be used to assess the viability of the gas capture process in MOFs modified with ILs or DESs and to get insight into the driving forces involved. Gas diffusion inside porous materials is another area where GCMC simulations might provide light. The performance of MOFs in gas separation applications may be evaluated by measuring their diffusivity and permeability, both of which are derived from the motion of gas molecules. GCMC simulations may be further developed to investigate the impact of various factors on the adsorption and separation characteristics of the MOFs. These parameters include, but are not limited to, temperature, pressure, and the composition of ILs or DESs. Because of this, materials with better gas-capture capacities may be designed and optimized.
Adsorption, selectivity, diffusion and thermodynamic properties of toxic gases and volatile organic compounds within micro-porous metal-organic frameworks functionalized with ionic liquids and deep eutectic solvents can all be studied with GCMC simulations, making them a valuable computational methodology. To study single component adsorption isotherm of structures like CO2, N2 and CH4 as well as binary mixtures such as CO2/N2 and CO2/CH4 mixtures and selectivity of these gases from pure ZIFs and composite materials GCMC simulations can be utilized. GCMC simulations may be used to investigate the single component adsorption isotherm of structures such as CO2, N2 and CH4, as well as binary mixtures such as mixes of CO2/N2 and CO2/CH4 and the selectivity of these gases from pure ZIFs and composite materials.
Conclusion
In conclusion, this review of the literature has provided a wideranging survey of the cutting-edge ideas behind the capture of Volatile Organic Compounds (VOCs) by combining micro-porous Metal-Organic Frameworks (MOFs) with Ionic Liquids (ILs) and Deep Eutectic Solvents (DESs). MOFs have come to the forefront in recent years owing to their unique ability to store and isolate gases. Synergistic effects and improved performance in VOC capture have been investigated by mixing MOFs with ILs and DESs. The study focuses on the progress made in this area, specifically how combining MOFs with ILs and DESs enhanced their adsorption capacity, selectivity and diffusion characteristics. There is hope that environmental and industrial problems may be solved by combining MOFs with ILs and DESs to better absorb volatile organic compounds. Nonetheless, we must recognize that we still face obstacles. Scalability and stability of the resultant MOF composites are discussed, along with other challenges such as adjusting the composition and characteristics of ILs and DESs. These difficulties might be seen as openings for new discoveries and innovations in the area of MOF-based VOC capture.
Future combination of MOFs with ILs and DESs shows promise for efficient collection of hazardous gases and volatile organic compounds. Exploring novel combinations of MOFs, ILs and DESs, as well as the effect of external elements like temperature, pressure, and composition, may contribute to future developments in this sector. Progressive initiatives in this area may aid in the creation of more efficient and sustainable solutions for VOC collection by tackling these problems and leveraging on the advantages of the integrated approach. This study sheds light on the current developments and potential applications of ionic liquid and deep eutectic solvent-based micro-porous metal-organic frameworks for the removal of hazardous gases and volatile organic chemicals. To propel innovation and growth in environmental and industrial applications, the combination of MOFs with ILs and DESs provides a viable road for the development of gas capture systems.
Future Prospective
There are interesting new directions that might be explored with the combination of Deep Eutectic Solvents (DESs) and Metal-Organic Frameworks (MOFs). Modern non-conventional solvents, such as DES solvents, have been shown to be useful as organic ligands in the building of Metal-Organic Frameworks (MOFs). Innovative and unique MOFs with broad applications in adsorption, catalysis, sensing, separation and gas capture/storage may be synthesized by combining the functional groups of DESs with the intrinsic sites of metal ions. More research is required to fully understand the potential of polymeric Deep Eutectic Solvents (PDESs) in the synthesis of Metal-Organic Frameworks (MOFs). Particle Depletion and Encapsulation Systems (PDESs) may be used to solve the problem of particle buildup in MOFs. They provide an opportunity to develop composite MOFs with improved stability and reactivity. Interestingly, PDESs may display diverse behaviors in bulk assemblies while having the same composition, which provides additional opportunities for modifying the characteristics of DES-based MOFs. Several more potential study avenues exist in addition to the aforementioned ones. First, MOFs built on DESs created by supramolecular self-assembly may undergo photodegradation\ opening the door to the creation of light-responsive MOFs with applications in environmental remediation.
Second, DES-based MOFs may be explored for their potential in photo-catalytic degradation of environmental toxicants, offering a potential solution to pollution problems. In order to fully comprehend and maximize MOF performance, it is necessary to look at how temperature and water affect their adsorption capabilities. Reactive hetero-atomic composites’ potential to alter the conformer of DES-based MOFs is also worthy of study. Finally, molecular dynamic simulations may help us learn more about the processes involved in making MOFs and their potential uses in the future. It cannot be overstated how crucial it is to have multidisciplinary teams work together to create DES-based MOFs. To combat the negative impacts of hazardous gases and strive towards a cleaner, more sustainable earth, a multidisciplinary team comprising of chemists, chemical engineers, waste management companies and ecological specialists is required. The potential for innovative visions in the field of micro-porous metal-organic frameworks based on DESs for capturing toxic gases and volatile organic compounds, leading to advances in environmental sustainability and industrial applications, can be realized through the pursuit of these future research directions and the promotion of collaborative efforts.
References
- Barber J (2009) Photosynthetic energy conversion: Natural and artificial. Chem Soc Rev 38(1): 185-196.
- Solomon S, Plattner GK, Knutti R, Friedlingstein P (2009) Irreversible climate change due to carbon dioxide emissions. Proceedings of the National Academy of Sciences 106(6): 1704-1709.
- Puga AV (2018) On the nature of active phases and sites in CO and CO2 hydrogenation catalysts. Catalysis Science & Technology 8(22): 5681-5707.
- Corma A, Garcia H (2013) Photocatalytic reduction of CO2 for fuel production: Possibilities and challenges. Journal of Catalysis 308: 168-175.
- Sakakura T, Choi JC, Yasuda H (2007) Transformation of Carbon Dioxide. Chemical Reviews 107(6): 2365-2387.
- Schneider J, Jia H, Muckerman JT, Fujita E (2012) Thermodynamics and kinetics of CO2, CO and H+ binding to the metal centre of CO2 Chem Soc Rev 41(6): 2036-2051.
- Gholampour N, Ezugwu CI, Rahmdele S, Gilanie AG, Verpoort F (2022) Adsorptive removal and catalytic performance of metal-organic frameworks containing mixed azolium-bipyridine ligand. Resources Chemicals and Materials 1(3-4): 201-210.
- Zhou HC, Long JR, Yaghi OM (2012) Introduction to Metal-organic frameworks. Chemical Reviews 112(2): 673-674.
- Amini M, Ramezani S, Anbari AP, Beheshti A, Gautam S, et al. (2018) Simple preparation of cuprous oxide nanoparticles for catalysis of azide-alkyne cycloaddition. Journal of Chemical Research 42(3): 166-169.
- Anbari AP, Delcheh SR, Heynderickx PM, Chaemcheun S, Zhuiykov S, et al. (2023) Green approach for synthesizing copper containing ZIFs as efficient catalysts for click chemistry. Catalysts 13(6): 1003.
- Morris RE, Wheatley PS (2008) Gas storage in nanoporous materials. Angewandte Chemie International Edition 47(27): 4966-4981.
- Prakash M, Jobic H, Ramsahye NA, Nouar F, Damasceno Borges D, et al. (2015) Diffusion of H2, CO2, and their mixtures in the porous zirconium based metal-organic framework MIL-140A(Zr): Combination of quasi-elastic neutron scattering measurements and molecular dynamics simulations. The Journal of Physical Chemistry C 119(42): 23978-23989.
- Ding M, Flaig R W, Jiang HL, Yaghi OM (2019) Carbon capture and conversion using metal-organic frameworks and MOF-based materials. Chemical Society Reviews 48(10): 2783-2828.
- Salles F, Jobic H, Maurin G, Koza MM, Llewellyn PL, et al. (2008) Experimental evidence supported by simulations of a very high H2 diffusion in metal organic framework materials. Physical Review Letters 100(24).
- Borges DD, Prakash M, Ramsahye N, Llewellyn P, Surblé S, et al. (2015) Computational exploration of the gas adsorption on the iron tetracarboxylate metal-organic framework MIL-102. Molecular Simulation 41(16-17): 1357-1370.
- Ghalei B, Sakurai K, Kinoshita Y, Wakimoto K, Isfahani A, et al. (2017) Enhanced selectivity in mixed matrix membranes for CO2 capture through efficient dispersion of amine-functionalized MOF nanoparticles. Nature Energy 2(7).
- Benzaqui M, Semino R, Menguy N, Carn F, Kundu T, et al. (2016) Toward an understanding of the microstructure and interfacial properties of PIMs/ZIF-8 mixed matrix membranes. ACS Applied Materials & Interfaces 8(40): 27311-27321.
- Semino R, Ramsahye NA, Ghoufi A, Maurin G (2015) Microscopic model of the metal-organic framework/polymer interface: A first step toward understanding the compatibility in mixed matrix membranes. ACS Applied Materials & Interfaces 8(1): 809-819.
- Kamalakannan S, Prakash M, Al-Mogren MM, Chambaud G, Hochlaf M (2019) Alkyl methyl imidazolium-based ionic liquids at the Au(111) surface: Anions and alkyl chain cations induced interfacial effects. The Journal of Physical Chemistry C 123(24): 15087-15098.
- Thomas A, Maiyelvaganan KR, Kamalakannan S, Prakash M (2019) Density functional theory studies on zeolitic imidazolate framework-8 and ionic liquid-based composite materials. ACS Omega 4(27): 22655-22666.
- Cadena C, Anthony JL, Shah JK, Morrow TI, Brennecke JF, et al. (2004) Why Is CO2 so soluble in imidazolium-based ionic liquids? Journal of the American Chemical Society 126(16): 5300-5308.
- Wu B, Liu WW, Zhang YM, Wang HP (2009) Do we understand the recyclability of ionic liquids? Chemistry A European Journal 15(8): 1804-1810.
- Srivatsav P, Bhargav BS, Shanmugasundaram V, Arun J, Gopinath KP, et al. (2020) Biochar as an eco-friendly and economical adsorbent for the removal of colorants (Dyes) from aqueous environment: A Review. Water 12(12): 3561.
- Li P, Cheng FF, Xiong WW, Zhang Q (2018) New synthetic strategies to prepare metal-organic frameworks. Inorganic Chemistry Frontiers 5(11): 2693-2708.
- Gilani AG, Sardroodi J, Verpoort F, Rahmdel S (2021) Experimental study and thermodynamic modeling of phase equilibria of systems containing cyclohexane, alcohols (C4 and C5), and deep eutectic solvents. Journal of Molecular Liquids 340: 117196.
- Du Q, Rao R, Bi F, Yang Y, Zhang W, et al. (2022) Preparation of modified zirconium-based metal-organic frameworks (Zr-MOFs) supported metals and recent application in environment: A review and perspectives. Surfaces and Interfaces 28: 101647.
- Luna JM, Sevillano JJ, Anta JA, Calero S (2013) Effect of room-temperature ionic liquids on CO2 separation by a Cu-BTC metal-organic framework. The Journal of Physical Chemistry C 117(40): 20762-20768.
- Zhang X, Bi F, Zhu Z, Yang Y, Zhao S, et al. (2021) The promoting effect of H2O on rod-like MnCeOx derived from MOFs for toluene oxidation: A combined experimental and theoretical investigation. Applied Catalysis B: Environmental 297: 120393.
- Wang Y, Bi F, Wang Y, Jia M, Tao X, et al. (2021) MOF-derived CeO2 supported Ag catalysts for toluene oxidation: The effect of synthesis method. Molecular Catalysis 515: 111922.
- Gilani AG, Dafrazi AA, Delcheh SR, Verpoort F (2020) Cyclopentanone-alkanediol systems: Experimental and theoretical study on hydrogen-bond complex formation. Industrial & Engineering Chemistry Research 59(40): 18318-18334.
- Abbott AP, Capper G, Davies DL, Munro HL, Rasheed RK, et al. (2001) Preparation of novel, moisture-stable, Lewis-acidic ionic liquids containing quaternary ammonium salts with functional side chains. Chemical Communications (19): 2010-2011.
- Kuo PJ, Lee CL, Wang JH, Hsieh SY, Huang SC, et al. (2017) Inhalation of volatile anesthetics via a laryngeal mask is associated with lower incidence of intraoperative awareness in non‐critically ill patients. PLoS One 12(10): e0186337.
- Ni Z, Yassar A, Antoun T, Yaghi OM (2005) Porous metal-organic truncated octahedron constructed from paddle-wheel squares and terthiophene links. Journal of the American Chemical Society 127(37): 12752-12753.
- Plechkova NV, Seddon KR (2008) Applications of ionic liquids in the chemical industry. Chem Soc Rev 37(1): 123-150.
- Abbott AP, Boothby D, Capper G, Davies DL, Rasheed RK (2004) Deep Eutectic solvents formed between choline chloride and carboxylic acids: Versatile alternatives to ionic liquids. Journal of the American Chemical Society 126(29)
: 9142-9147. - Li S, Huo F (2015) Metal-organic framework composites: from fundamentals to applications. Nanoscale 7(17): 7482-7501.
- Li W, Henke S, Cheetham AK (2014) Research update: Mechanical properties of metal-organic frameworks-Influence of structure and chemical bonding. APL Materials 2(12): 123902.
- Li J, Miao P, Chen KJ, Cao JW, Liang J, et al. (2020) Highly effective electromagnetic wave absorbing Prismatic Co/C nanocomposites derived from cubic metal-organic framework. Composites Part B: Engineering 182: 107613.
- Liang Q, Jin H, Wang Z, Xiong Y, Yuan S, et al. (2019) Metal-organic frameworks derived reverse-encapsulation Co-NC@Mo2C complex for efficient overall water splitting. Nano Energy 57: 746-752.
- Chen Y, Tang S (2019) Solvothermal synthesis of porous hydrangea-like zeolitic imidazole framework-8 (ZIF-8) crystals. Journal of Solid State Chemistry 276: 68-74.
- Carné A, Carbonell C, Imaz I, Maspoch D (2011) Nanoscale metal-organic materials. Chem Soc Rev 40(1): 291-305.
- Mansour RB, Qasem NA, Habib MA (2018) Adsorption characterization and CO2 breakthrough of MWCNT/Mg-MOF-74 and MWCNT/MIL-100(Fe) composites. International Journal of Energy and Environmental Engineering 9(2): 169-185.
- Han T, Xiao Y, Tong M, Huang H, Liu D, et al. (2015) Synthesis of CNT@MIL-68(Al) composites with improved adsorption capacity for phenol in aqueous solution. Chemical Engineering Journal 275: 134-141.
- Bolisay LD, Culver JN, Kofinas P (2007) Optimization of virus imprinting methods to improve selectivity and reduce nonspecific binding. Biomacromolecules 8(12): 3893-3899.
- Lin Z, Li Y, Slawin AM, Morris RE (2008) Hydrogen-bond-directing effect in the ionothermal synthesis of metal coordination polymers. Dalton Transactions (30): 3989.
- Parnham ER, Morris RE (2006) Ionothermal synthesis using a hydrophobic ionic liquid as solvent in the preparation of a novel aluminophosphate chain structure. Journal of Materials Chemistry 16(37): 3682.
- Khan NA, Hasan Z, Jhung SH (2013) Ionic liquids supported on metal-organic frameworks: Remarkable adsorbents for adsorptive desulfurization. Chemistry A European Journal 20(2): 376-380.
- Xie Y, Zhang Z, Jiang T, He J, Han B, et al. (2007) CO2 cycloaddition reactions catalyzed by an ionic liquid grafted onto a highly cross-linked polymer matrix. Angewandte Chemie International Edition 46(38): 7255-7258.
- Shi F, Deng Y, SiMa T, Peng J, Gu Y, et al. (2003) Alternatives to phosgene and carbon monoxide: Synthesis of symmetric urea derivatives with carbon dioxide in ionic liquids. Angewandte Chemie International Edition 42(28): 3257-3260.
- Zhang Z, Xie Y, Li W, Hu S, Song J, et al. (2008) Hydrogenation of carbon dioxide is promoted by a task-specific ionic liquid. Angewandte Chemie International Edition 47(6): 1127-1129.
- Ghazali Z, Suhaili N, Tahari MN, Yarmo MA, Hassan NH, et al. (2020) Impregnating deep eutectic solvent choline chloride: Urea: Polyethyleneimine onto mesoporous silica gel for carbon dioxide capture. Journal of Materials Research and Technology 9(3): 3249-3260.
- Li H, Zhang J, Yi J, Luo J, Zhu S, et al. (2019) Sn-based deep eutectic solvents assisted synthesis of Sn and SnO2 supported hexagonal boron nitrides for adsorptive desulfurization. Chemical Engineering Research and Design 144: 11-18.
- Chen S, Zhang J, Wu T, Feng P, Bu X (2009) Multiroute synthesis of porous anionic frameworks and size-tunable extra framework organic cation-controlled gas sorption properties. Journal of the American Chemical Society 131(44): 16027-16029.
- Wang Y, Xu Y, Ma H, Xu R, Liu H, et al. (2014) Synthesis of ZIF-8 in a deep eutectic solvent using cooling-induced crystallisation. Microporous and Mesoporous Materials 195: 50-59.
- Jiang W, Dong L, Li H, Jia H, Zhu L, et al. (2019) Magnetic supported ionic liquid catalysts with tunable pore volume for enhanced deep oxidative desulfurization. Journal of Molecular Liquids 274: 293-299.
- Luo L, Wu Z, Wu Z, Liu Y, Huang X, et al. (2022) Role of structure in the ammonia uptake of porous polyionic liquids. ACS Sustainable Chemistry & Engineering 10(13): 4094-4104.
- Luo L, Li J, Chen X, Cao X, Liu Y, et al. (2022) Superhigh and reversible NH3 uptake of cobaltous thiocyanate functionalized porous poly ionic liquids through competitive and cooperative interactions. Chemical Engineering Journal 427: 131638.
- Li JR, Kuppler RJ, Zhou HC (2009) Selective gas adsorption and separation in metal-organic frameworks. Chemical Society Reviews 38(5): 1477.
- Xue W, Li Z, Huang H, Yang Q, Liu D, et al. (2016) Effects of ionic liquid dispersion in metal-organic frameworks and covalent organic frameworks on CO2 capture: A computational study. Chemical Engineering Science 140: 1-9.
- Yang S, Callear SK, Cuesta AJ, David WI, Sun J, et al. (2011) Pore with gate: Modulating hydrogen storage in metal-organic framework materials via cation exchange. Faraday Discussions 151: 19.
- Li Y, Tian X, Jiang W, Wu P, Li HS, et al. (2021) Amino and triazole-containing metal-organic frameworks for highly efficient CO2 Chemical Communications 57(82): 10803-10806.
- McDonald TM, Lee WR, Mason JA, Wiers BM, Hong CS, et al. (2012) Capture of carbon dioxide from air and flue gas in the alkylamine-appended metal-organic framework mmen-Mg2(dobpdc). Journal of the American Chemical Society 134(16): 7056-7065.
- Nugent P, Belmabkhout Y, Burd SD, Cairns AJ, Luebke R, et al. (2013) Porous materials with optimal adsorption thermodynamics and kinetics for CO2 Nature 495(7439): 80-84.
- Roohi H, Jahantab M, Delcheh SR, Khoshakhlagh BP (2014) Chemical functionalization of boron nitride nanotube via the 1,3-dipolar cycloaddition reaction of azomethine ylide: A quantum chemical study. Structural Chemistry 26(3): 749-759.
- Aad G, Abbott B, Abbott DC, Abdinov O, Abud AA, et al. (2020) Measurement of differential cross sections for single diffractive dissociation in collisions using the ATLAS ALFA spectrometer. Journal of High Energy Physics 2020(2).
- Thomas A, Maiyelvaganan KR, Kamalakannan S, Prakash M (2019) Density functional theory studies on zeolitic imidazolate framework-8 and ionic liquid-based composite materials. ACS Omega 4(27): 22655-22666.
- Galvelis R, Slater B, Cheetham AK, Draznieks CM (2012) Comparison of the relative stability of zinc and lithium-boron zeolitic imidazolate frameworks. CrystEngComm 14(2): 374-378.
© 2023 Shima Rahmdel Delcheh. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






