- Submissions

Full Text
Progress in Petrochemical Science
Preparation and Characterization of Biodiesel by Catalyst (CaO/NaF) and Study of the Possibility of Blending with Petroleum Diesel Fuel
Mayas Saad1*, Baraa Siyo2 and Hussam Alrakkad3
Department of chemistry, Faculty of science, Tishreen University, Syria
*Corresponding author:Mayas Saad, Department of chemistry, Faculty of science, Tishreen University, Syria
Submission: August 22, 2023;Published: September 07, 2023

ISSN 2637-8035Volume5 Issue5
Abstract
In this study, biodiesel was prepared by transesterification reaction of waste cooking oils using a heterogeneous catalyst (CaO/NaF) prepared from waste eggshells, which can be reused for several cycles, and the optimal conditions for the preparation were studied. This work aims to prepare and evaluate biodiesel according to the quality requirements specified in international standards and to study the possibility of mixing it with petroleum diesel fuel at the ratios of 5%, 20%. Biodiesel was obtained with a yield of 94.5% under the optimum reaction conditions: mol oil to alcohol ratio (1:9), catalyst ratio (4% of oil weight), temperature 60 °C and reaction time of 120 minutes. The pure biodiesel (B100) was characterized by gas chromatography coupled with mass spectrometry (GC.MS) and infrared spectroscopy (FTIR). The physical and chemical characteristics of the prepared biodiesel B100 and biodiesel samples mixed with petroleum diesel (B5, B20) were also studied. The results showed that the physical and chemical specifications of biodiesel samples (B100, B5, B20) were in accordance with the American (ASTM) and European (EN) standards.
Keywords: Biodiesel; Transesterification; Egg shells; Waste cooking oils; Sodium fluoride; Petroleum diesel
Nomenclature: ASTM: American Society for Testing Materials; EN: European Standards; B: Biodiesel; B100: (100% biodiesel); B20: (80% diesel and 20% biodiesel); B5: (95% diesel and 5% biodiesel); FFA: Free Fatty Acid; WCO: Waste Cooking Oil; FAME: Fatty Acid Methyl Ester
Introduction
Increased population growth, change in lifestyles, urbanization, and many other reasons, have precipitated an upsurge in the global demand for energy in the last few decades. This increased demand for energy, environmental concerns, depletion in fossil fuel reserves, instability in the global oil price, continuous increases in the price of fossil-based petroleum products, high cost of exploration and unacceptable combustion and performance of fossil-based fuels in internal combustion engines has led to an urgent search for sustainable alternative fuel to substitute fossil-based fuel [1]. In the last decade, the world has witnessed many changes in the energy field where many companies around the world created new strategies based on reducing the environmental impact and achieving competitive prices of biofuel compared with fossil fuel. The new form of energy has several features. It requires lower cost of infrastructure and lower environmental impact since it will replace a big share of demand for fossil fuel. In addition, it reduces effects of greenhouse gases due to less SOx, NOx and CO2 gases emissions compared with fossil fuel production.
At the same time the capability to produce it from different raw agriculture materials, edible and non-edible oil is increasing. However, it is less than expected due to bad weather drought affect and growth of global [2,3]. Today biodiesel compared with petroleum is considered an environmentally friendly fuel due to low carbon dioxide emissions, biodegradable fuel, high cetane number, high combustion efficiency, lower aromatic and sulfur content in comparison to petroleum diesel, making the biodiesel a competitive fuel in the market [4,5]. Biodiesel production aims to get good qualities and quantities by choosing suitable and cheap feedstock such as virgin vegetable oils, used cook oils and animal fats. Other types of edible vegetable oil can be used for instance; soybean oil, sunflower, palm oil, canola and peanut oil or even non-edible oils such as sea mango, jatropha, rubber seed and pongamia pinnata [6]. Direct use of the oil causes poor fuel atomization in injection, gum formation, deposition of coke in engine, oxidation and polymerization of fatty acids due to the high viscosity of the oil. The combustion efficiency decreases because of insufficient mixing of the fuel with air leading to higher emission of hydrocarbons.
By producing biodiesel, the physical properties of the oils are enhanced making the biodiesel a reliable, safe fuel to use for diesel engines. Biodiesel uses to be blended with conventional diesel in different percentages [7,8]. There are various production procedures for development of biodiesel, namely microemulsion, pyrolysis and transesterification. Transesterification process is mostly used in the industrial production of biodiesel due to its cost affordability. Among the mentioned procedures, transesterification is the most common, economic, high conversion yield and appropriate method for biodiesel production [9,10]. Biodiesel is produced through transesterification reactions of vegetable oil and animal fats with alcohol. Methanol or ethanol are usually the alcohols for biodiesel preparation. The reaction is facilitated with a suitable catalyst either homogeneous or heterogeneous [11]. The use of homogeneous alkali or acid catalysts has some hurdles associated with them. These include excessive consumption of reactants, soap formation, environmental pollution and separation difficulty, which may add to the cost of biodiesel production [12].
The use of heterogeneous solid catalyst has been proposed as a solution to various drawbacks linked to homogeneous catalysts. The development of a calcium oxide catalyst derived from eggshell waste biomass has been receiving attention because it is reusable, recoverable, ecofriendly, not corrosive and produces no soap [13]. The competition of producing biodiesel related with raw materials cost is high since the production process uses edible oil. Moreover, the conflict on food prices is currently high. For these reasons, the use of cheap non-edible vegetable oils or waste cooking oils decreases the cost of the raw materials [14]. Biodiesels produced from vegetable oils and animal fats have been shown to have a higher viscosity than diesel and can be used as a fuel in diesel engines without any significant breakdown in performance [15].
Its usage in diesel engines produces fewer amounts of smoke, noise, carbon monoxide, sulfur-containing compounds and polyaromatic hydrocarbons compared to fossil fuels [16]. The main aim of the present study is to produce and characterize biodiesel through transesterification process using low value triglyceride resources such as waste edible oils in the presence of a catalyst (Cao/NaF) prepared from eggshell waste as a heterogeneous catalyst that can be reused for several production cycles. After the preparation and purification of the biodiesel, the biodiesel is mixed with petroleum diesel produced in Syria in different ratios. The physical properties of the mixtures, such as density, kinematic viscosity, flash point and pour point are measured according to international standards and these properties compared to standard conditions. Finally, the best ratio of biodiesel/diesel is obtained.
Experimental
Materials
The materials used in this study were: Waste Cooking Oil (WCO) was collected from nearby restaurants from Tishreen university, methanol (anhydrous, 99.6%), distilled water, eggshells, sodium fluoride.
Catalyst preparation
The catalyst was prepared using the wet impregnation method, calcium oxide was prepared from eggshells, where the eggshells were washed well with tap water to clean them of residue, then washed with distilled water, dried at 110 oC for 3 hours, and calcined at 900 oC for 3 hours. Then the resulting CaO is loaded with NaF at a rate of 20%, whereby 10g of calcium oxide obtained from eggshells is taken, 2g of NaF is dissolved in the least possible amount of distilled water, then added to CaO so that a wet powder of calcium oxide is formed according to the ratio. The solution is left for 24 hours at laboratory temperature, dried at 105 oC for 5 hours, then calcined at 900 oC for 4 hours.
Figure 1:The transesterification of vegetable oil or fat with methanol alcohol.
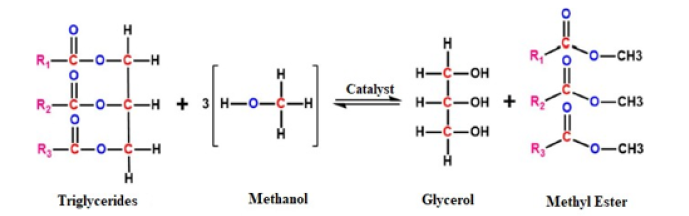
Results and Discussion
Optimization of reaction conditions
Figure 2:Separation of glycerol phase from biodiesel phase in a lab separating funnel.

Figure 3:Effect of methanol/oil molar ratio (a), catalyst amount (b), reaction time (C), and temperature (d) on the biodiesel yield.
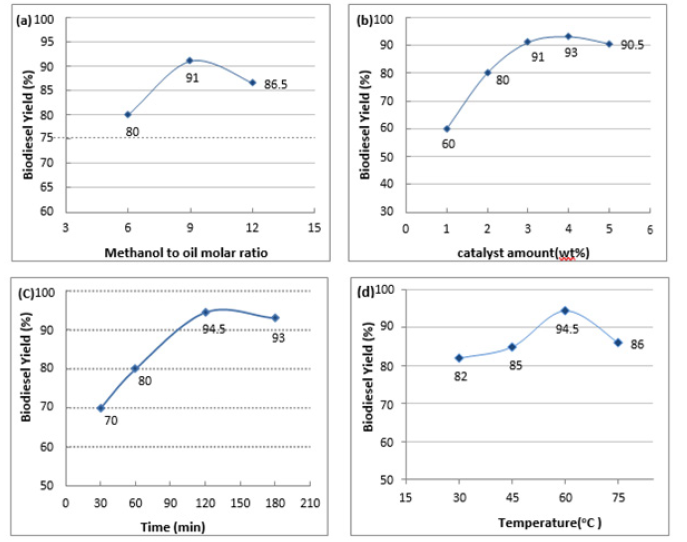
The effect of changing the reaction parameters as: molar ratio of methanol/oil, amount of catalyst, reaction time and reaction temperature on the transesterification of waste oil were investigated see (Figure 3).
Effect of molar ratio of oil to methanol on biodiesel yield
The study of transesterification reactions of waste cooking oil was performed using different methanol to oil ratios (1:6, 1:9, 1:12) in the presence of 3% by weight of a CaO/NaF catalyst for 3 hours at 60 °C. The experimental results shown in Figure 3a indicate that the molar ratio of methanol to oil has a significant impact on the biodiesel yield. The biodiesel yield was increased with the molar ratio 91% yield was reported at 1:9 molar ratio of oil to the methanol. The excess of methanol is necessary because it can increase the rate of metanalysis. The high amount of methanol promoted the formation of methoxy species on the catalyst surface, leading to a shift in the equilibrium towards forward direction, thus increasing the rate of biodiesel conversion [18]. However, the yield was slightly reduced when the ratio of oil to methanol was higher than 1:9. The biodiesel yield was only 86.5% at 1:12 oil to methanol molar ratio. Further increase in oil-to-methanol ratio after optimal ratio of 1:9 would lead to a reduction of the biodiesel yield. This is due to excessive methanol beyond the optimal point which does not promote the reaction. The glycerol which is a by-product of the reaction would largely dissolve in the excessive methanol and subsequently inhibit the reaction of methanol to reactants and catalyst, thus interfering with the separation of glycerin, which in turn lowers the conversion by shifting the equilibrium in the reverse direction [19].
Effect of catalyst amount on biodiesel yield
The effect of CaO/NaF catalyst amount used on the transesterification reaction of waste cooking oil was investigated using catalyst amount of 1-5 % by weight for 3 hours at 60 °C and an oil to methanol ratio of 1:9. Catalyst concentration plays an important role in optimizing the yield of transesterification reaction. From Figure 3b, it can be seen that biodiesel yield increases with the increase of catalyst concentration from 1% w/w to 4% w/w. This can be assigned to the increase in the present active catalytic sites of the catalyst with increasing the amount [20]. The optimal catalyst concentration was determined to be 4% w/w CaO/NaF catalyst with a biodiesel yield of 92%. The excess catalyst has slightly reduced the biodiesel yield due to soap formation. Further, this can be referred to the increase in the viscosity of the reaction medium under the formation of dense slurry from the catalyst powder and the used oil, which resulted in a difficulty in the homogeneous mixing of the reactants and consequently decreases in the interaction between the catalyst and reaction component [21].
Effect of reaction time on biodiesel yield
Figure 3c shows the effect of different reaction time from 0.5h to 3h on the conversion of waste cooking oil into biodiesel. At fixed conditions of 1:9 oil to methanol ratio, 60 °C reaction temperature, and 4% catalyst amount, the biodiesel yield showed a gradual increase with increasing the transesterification time from 0.5 to 2h. Then, the conversion efficiency was slightly decreased with increasing the reaction time from 2 to 3h. Production of biodiesel was rapid until the reaction reached equilibrium. Beyond the optimal point, the reaction starts to reverse in a backward direction towards reactants. While reversing of the behavior after a specific reaction time can be attributed to the reversible nature of the conversion reaction of oils into biodiesel [22,23]. CaO catalyst has a tendency to adsorb products when reactant was lack [24]. Therefore, too long reaction time also reduces the biodiesel yield as the CaO catalyst can absorb the product. Hence it is important to identify the optimum reaction time for the transesterification reaction. In this case, the optimum reaction time was 2 hours with a biodiesel yield of 94.5%.
Effect of reaction temperature on biodiesel yield
It was reported that increasing the transesterification reaction temperature has a direct reflection on the transesterification reaction subsequently the conversion of the oil into biodiesel [25]. Figure 3d shows the biodiesel yield from transesterification of waste cooking oil at different reaction temperatures from 30 °C to 75 °C using 4% by weight of the catalyst for 2h at a 1:9 oil to methanol ratio. The biodiesel yield increases with the reaction temperature until an optimal point of 60 °C with a biodiesel yield of 94.5%. Beyond this, the yield decreased abruptly to 80% at 75 °C. This can be attributed to the evaporation of the methanol by increasing the temperature which can reduce the methanol: oil molar ratio during the conversion reaction and also due to the reverse behavior of the transesterification reaction [25]. Initially, some thermal energy was needed for the transesterification as the reaction was endothermic [26]. Since the reaction mixture constitutes a three-phase system (oil-methanol-catalyst), the thermal energy was sufficiently needed to overcome the diffusion resistance between different phases [27]. However, high temperatures are not preferred. As the temperature increases and reaches the boiling point of methanol, the methanol will quickly vaporize and form a large number of bubbles, which inhibits the reaction on the two-phase interface and thus decreases the biodiesel yield [28].
Reusability of the catalyst
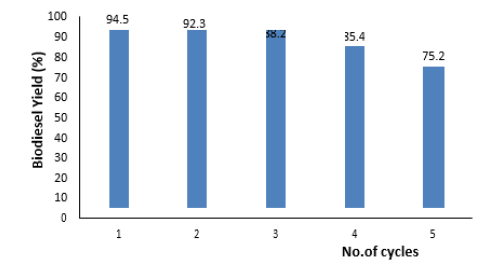
The ability to reuse catalysts not only reduces costs but also makes the complex separation process more stable, efficient and recyclable. A catalyst was necessary for biodiesel synthesis. From an economic point of view, more reusable catalyst is required, particularly for industrial applications. To evaluate the reusability, the solid catalyst was separated through filtration followed by centrifugation. The used catalyst after each run washed several times with a mixture of methyl alcohol and n-hexane (1:1 V:V), then reactivated at 800 °C for 3h. The suitability of CaO/NaF composite for several runs of transesterification of waste cooking oil into biodiesel was represented in Figure 4, for 5 runs at the preestimated optimum reaction conditions (2h, 1:9 oil to methanol ratio, 4% catalyst amount, 60 °C). The catalytic activity of the synthetic catalyst decreased with increasing the conversion runs. This is closely related to the decreasing of the active catalytic sites under the sequential use of the catalyst due to the precipitation of the insoluble by-products on its surface, e.g., glycerol [29]. The biodiesel yield decreased by 94.5%, 92.3%, 88.2%, 85.4%, and 75.2% with reusing the catalyst from 5 runs.
Figure 4:Effect of catalyst run cycle on FAME yield.
Comparison of biodiesel yield from different heterogeneous catalyst
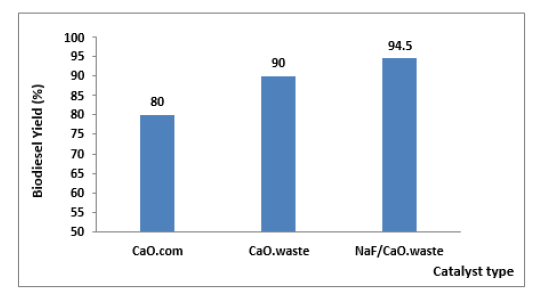
Figure 5 shows the biodiesel yield for the transesterification of waste cooking oils using catalysts (eggshell-derived calcium oxide, eggshell-derived calcium oxide loaded with 25% NaF, commercial calcium oxide) using optimized reaction conditions. From the experiment, it was determined that the optimal conditions for the transesterification of waste cooking oils with CaO/NaF prepared from eggshells were 1:9 (oil: methanol), 4% w/w catalyst amount, 60 °C reaction temperature, and 2h reaction time. These optimal conditions gave the highest biodiesel yield of 94.5%. Then the optimal conditions were then used for transesterification of waste cooking oil using calcium oxide prepared from eggshells and commercial calcium oxide as a catalyst and the achieved biodiesel yield was 90% and 80%, respectively. The result showed that the calcium oxide synthesized from eggshells is more reactive than the commercial compound, which indicates the possibility of using eggshells as a low-cost catalyst for the production of biodiesel. An important effect of loading with sodium ions on the yield is noted with a conversion rate of 4.5% of the conversion rate; It results from enhancing the basic properties of the catalyst when loaded with sodium ions, which makes it more efficient in catalyzing transesterification reactions. The biodiesel production from the transesterification of waste cooking oils in this study was higher compared to other reported studies, a biodiesel production of 91.17% was observed for the transesterification of castor oil using calcium oxide derived from mussel shell [30].
Figure 5:Comparison of CaO catalyst performance for FAME production.
Biodiesel confirmation by warn quits 3/27
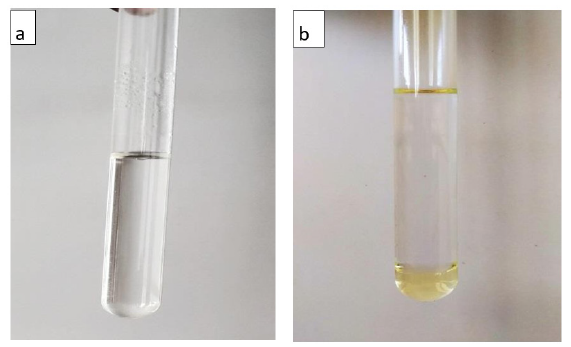
Warn quits 3/27 it is the test that is useful in knowing the degree of conversion of triglycerides to biodiesel. This test is important and inexpensive compared to analyzes using gas chromatography. This test can be done during the experiment to find out the time needed for complete transformation and it can be done after the reaction to compare it with other samples using different catalysts [31]. 3mL of the produced biodiesel was dissolved in 27mL of methanol and left undisturbed for 30 min to find the unconverted glycerides in the biodiesel after transesterification process, Since FAME is soluble in polar solvent it will get completely dissolved in methanol Figure 6a, the nonpolar unconverted oils remain settle down as precipitate Figure 6b [32].
Figure 6:(a)Biodiesel sample with methanol, (b) Oil sample with methanol.
FTIR spectra for oil and biodiesel
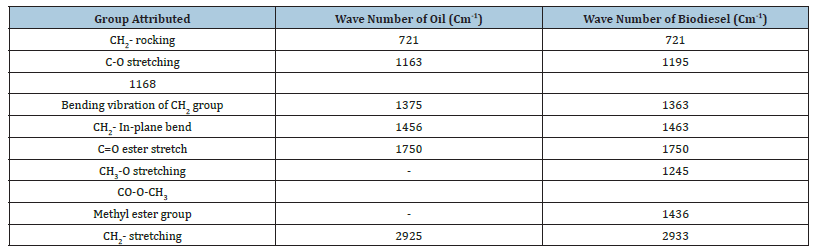
Oil and biodiesel were analyzed by FTIR as shown in Figures 7 & 8, the results shown in Tables 1. The IR spectra of the oil and biodiesel samples are very similar to each other because the reaction simply consists of removing glycerin and substitution of a methyl group in the hydrocarbon chain. The only significant difference can be seen in the bands lying between 1000-1500cm- 1. Particularly, the bands at 1163 and 1097cm-1 in the oil sample correspond to the expansion vibration of the (C-O) group bound to (CH2-) which shifts to 1168cm-1 in the biodiesel sample. However, new bands at 1195 and 1436cm-1 were observed in the biodiesel sample associated with the bending and oscillation vibrations of the (CH3- O) group which are not present in the spectrum of the oil. The bands in the range of 1435-1460cm-1 in the biodiesel spectrum are due to the asymmetric vibration of methane (CH3), indicating the conversion of the used oil to biodiesel as found by previous studies [33].
Figure 7:FTIR for oil sample.
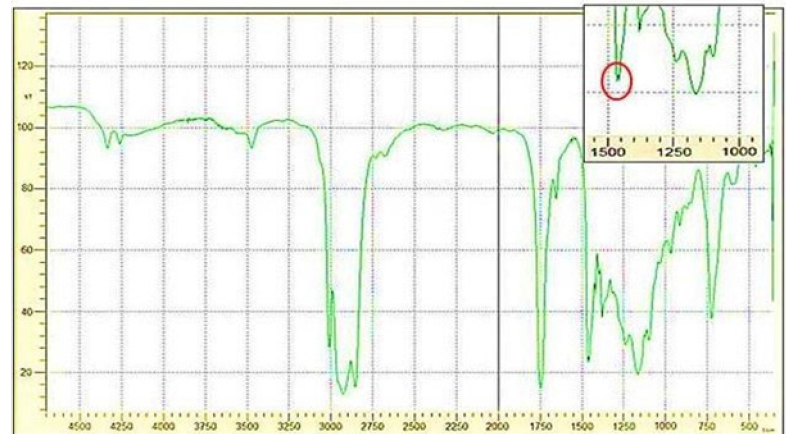
Figure 8:FTIR for biodiesel sample.
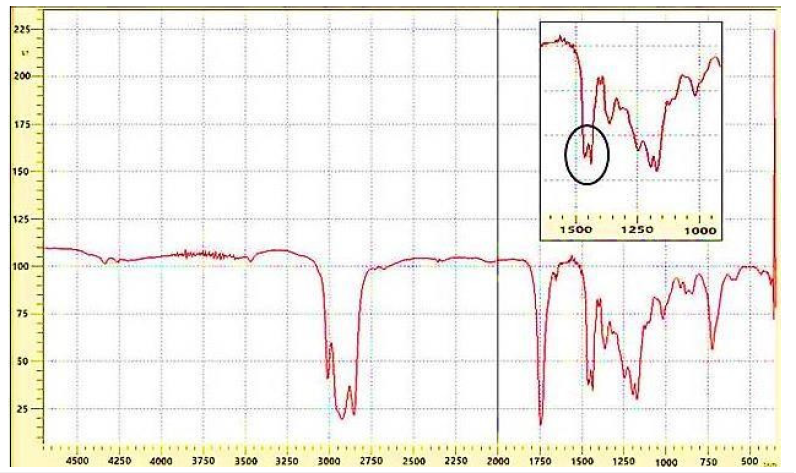
Table 1:FTIR wave numbers for different peaks of oil and biodiesel.
GC-MS analysis of biodiesel (FAME)
The chemical composition of biodiesel prepared from waste cooking oils was determined using Gas chromatography-mass spectrometry by comparing the mass spectra produced for each vertex of the GC.MS chromatogram with the mass spectra available in the library (NIST) available in the device. After analyzing the biodiesel, 9 components were identified through the GC.MS chromatogram shown in Figure 9 & Table 2 shows the biodiesel components. The results shown in the Table 2 indicate that the main components of biodiesel, according to their percentages, are as follows: 9-12- Octadecadienoic acid methyl ester (E, E) (79.6%) Figure 10, Hexadecenoic acid, methyl ester (11.5%), Methyl stearate (7.1%).
Table 2:The composition of Fatty Acid Methyl Ester (FAME) in biodiesel from waste cooking oil.
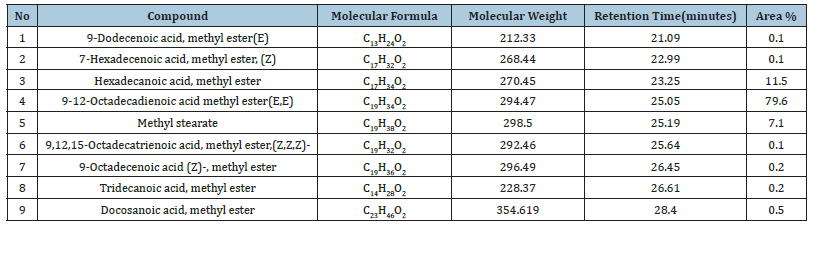
Figure 9:GC-MS chromatograms of the prepared biodiesel sample.
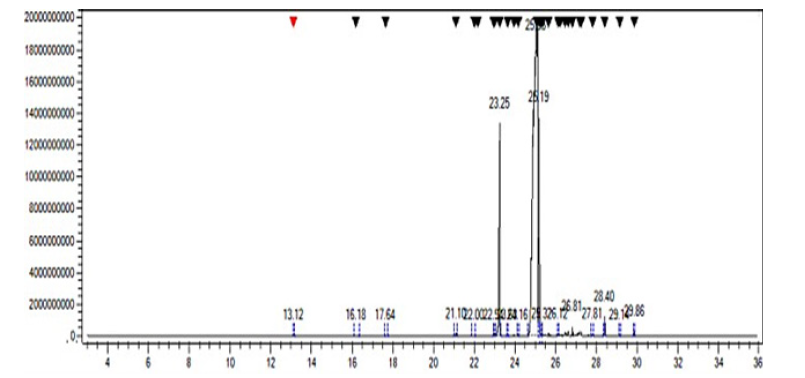
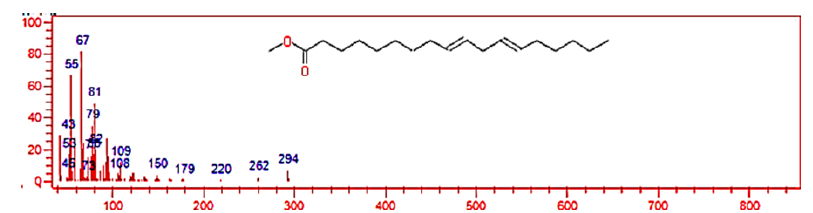
Figure 10:Mass spectrum of 9-12-Octadecadienoic acid methyl ester(E,E).
Studying the possibility of mixing prepared biodiesel with petroleum diesel
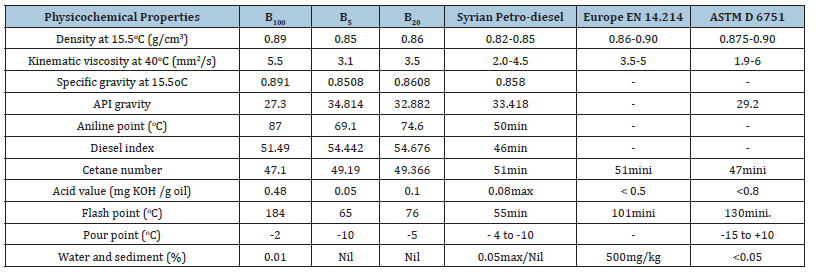
Two samples of biodiesel mixed with petroleum diesel at proportions (5%, 20%) were prepared and their physical and chemical properties were studied and compared to the American (ASTM) and European (EN) standards. The results shown in Table 3 indicated that the physical and chemical properties of pure biodiesel (B100) and the two samples of biodiesel mixed with Syrian diesel (B5, B20) were in conformity with the internationally proven specifications [34,35], and thus concludes that it is possible to mix biodiesel with Syrian diesel fuel. The results also indicate that the mixing ratio B20 is the best in terms of improving the values of density, kinematic viscosity, cetane number and flash point.
Table 3:Physical and chemical properties of biodiesel samples (B100, B5, B20) in comparison with (ASTM) and (EN).
Comparison of CaO/NaF catalyst with other catalysts
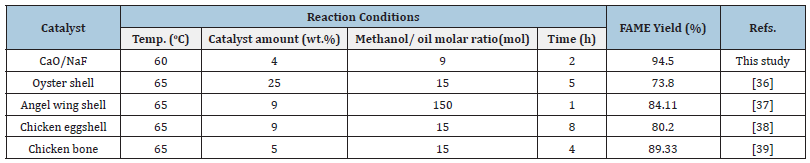
Table 4 contains a comparison of the present catalyst with some Ca based catalysts. Although the biodiesel yield achieved with other catalysts in Table 4 was lower than the yield achieved in our current study, the reaction conditions were higher. When using CaO/NaF catalysts, a higher yield was obtained at lower temperatures, lower catalyst amount and in a shorter time. This means lower production costs, which is the desired situation in biodiesel production [36- 39].
Table 4:Comparison of catalytic activity of CaO/NaF catalyst with reported Ca-based solid alkali catalysts.
Conclusion
The potential to produce biodiesel from waste cooking oil was economical and reduced environmental pollution associated with petroleum-based diesel. Apart from biodiesel production, these environmentally friendly waste-derived heterogeneous catalysts have the potential to be effective catalysts in a variety of chemical transformations. The study shows that waste material can be converted into a valuable product while using minimal energy during the transesterification reaction. Moreover, the waste eggshell contains a high percentage of CaO when it is calcined, which can be used as a substitute catalyst for biodiesel production. In comparison to other methods of catalyst preparation the heterogeneous catalyst formulation from eggshells is less expensive. The results obtained from this study showed that the heterogeneous catalyst (CaO/NaF) is effective for converting waste cooking oil into biodiesel with a yield of 94.5% under the following reaction conditions; The ratio of methanol to oil is (1:9) mol, the amount of catalyst (4% of the weight of the oil), the reaction temperature is 60 °C, and the reaction time is 2h. High purity biodiesel was obtained. The produced biodiesel was characterized and compared to international standards and the results were in compliance with American (ASTM) and European (EN) standards. Reusability testing shows that the catalyst (CaO/NaF) derived from eggshells can be reused up to 5 times. The results showed the possibility of mixing biodiesel with petroleum diesel fuel, and the results showed that the mixing ratio B20 was better than the mixing ratio B5. Based on the result of this study, researchers should focus on a low-grade feedstock like WCO, affordable and effective heterogeneous catalysts, as a novel approach to replace non-economical catalysts, the photocatalytic activity, stability, and surface morphology of the synthesized CaO/ NaF require further investigation through advanced instrumental characterization such as Thermogravimetric Analysis (TGA) for thermal stability, Scanning Electron Microscopy (SEM) for surface morphology, and Energy Dispersive X-Ray (EDX) spectroscopy for chemical characterization of a catalyst.
References
- Ouanji F, Kacimi M, Ziyad M, Puleo F, Liotta LF (2017) Production of biodiesel at small-scale (10L) for local power generation. International Journal of Hydrogen Energy 42(13): 8914-8921.
- Khurshid, Samir Najem Aldeen (2014) Biodiesel production by using heterogeneous catalyst.
- Tuomo P, Markku K, Erja T, Niko T (2104) Organic waste streams in energy and biofuel production.
- Vicente G, Martinez M, Aracil Jose (2004) Integrated biodiesel production: A comparison of different homogeneous catalysts systems. Bioresource Technology 92(3): 297-305.
- Martini N, Schell JS (1998) Plant oils as fuels. Present state of science and future development. Proceedings.
- Gui MM, Lee KT, Bhatia S (2008) Feasibility of edible oil vs. non-edible oil vs. waste edible oil as biodiesel feedstock. Energy 33(11): 1646-1653.
- Kuniyil M, Kumar JV, Adil SF, Assal ME, Shaik MR, et al. (2021) Production of biodiesel from waste cooking oil using ZnCuO/N-doped graphene nanocomposite as an efficient heterogeneous catalyst. Arabian Journal of Chemistry 14(3): 102982.
- Zhao Y, Wang G, Zhang L, Chang Y, Hao Y (2021) Converting waste cooking oil to biodiesel in China: Environmental impacts and economic feasibility. Renewable and Sustainable Energy Reviews 140: 110661.
- Keihani M, Esmaeili H, Rouhi Parham (2018) Biodiesel production from chicken fat using nano-calcium oxide catalyst and improving the fuel properties via blending with diesel. Physical Chemistry Research 6(3): 521-529.
- Baskar G, Soumiya S (2016) Production of biodiesel from castor oil using iron (II) doped zinc oxide nanocatalyst. Renewable Energy 98: 101-107.
- Patel R, Patel S (2017) Renewable hydrogen production from butanol: A review. Clean Energy 1(1): 90-101.
- Betiku E, Ajala SO (2014) Modeling and optimization of Thevetia peruviana (yellow oleander) oil biodiesel synthesis via Musa paradisiacal (plantain) peels as heterogeneous base catalyst: A case of artificial neural network vs. response surface methodology. Industrial Crops and Products 53: 314-322.
- Savaliya ML, Bhakhar MS, Dholakiya BZ (2016) Cutting cost technology for the preparation of biodiesel using environmentally benign and cheaper catalyst. Catalysis Letters 146(11): 2313-2323.
- Kibazohi O, Sangwan RS (2011) Vegetable oil production potential from Jatropha curcas, Croton megalocarpus, Aleurites moluccana, Moringa oleifera and Pachira glabra: Assessment of renewable energy resources for bio-energy production in Africa. Biomass Bioenergy 35: 1352-1356.
- Seffati K, Honarvar B, Esmaeili H, Esfandiari N (2019) Enhanced biodiesel production from chicken fat using CaO/CuFe2O4 nanocatalyst and its combination with diesel to improve fuel properties. Fuel 235: 1238-1244.
- Borges ME, Díaz L (2012) Recent developments on heterogeneous catalysts for biodiesel production by oil esterification and transesterification reactions: A review. Renewable and Sustainable Energy Reviews 16(5): 2839-2849.
- Ahmed M, Ullah K, Khan MA, Ali S, Zafar M, et al. (2011) Quantitative and qualitative analysis of sesame oil biodiesel. Energy Source, Part A: Recovery Utilization and Environmental Effects 33(13): 1239-1249.
- Buasri A, Chaiyut N, Loryuenyong V, Worawanitchaphong P, Trongyong S (2013) Calcium oxide derived from waste shells of mussel, cockle and scallop as the heterogeneous catalyst for biodiesel production. The Scientific World Journal 2013: 460923.
- Lim BP, Maniam GP, Hamid SA (2009) Biodiesel from adsorbed waste oil on spent bleaching clay using CaO as a heterogeneous catalyst. European Journal of Scientific Research 33(2): 347-357.
- Negm NA, Sayed GH, Yehia FZ, Habib OI, Mohamed EA (2017) Biodiesel production from one-step heterogeneous catalyzed process of Castor oil and Jatropha oil using novel sulphonated phenyl silane montmorillonite catalyst. Journal of Molecular Liquids 234: 157-163.
- Negm NA, Sayed GH, Habib OI, Yehia FZ, Mohamed EA (2017) Heterogeneous catalytic transformation of vegetable oils into biodiesel in one-step reaction using super acidic sulfonated modified mica catalyst. Journal of Molecular Liquids 237: 38-45.
- Abukhadra MR, Dardir FM, Shaban M, Ahmed EA, Soliman MF (2018) Spongy Ni/Fe carbonate-fluorapatite catalyst for efficient conversion of cooking oil waste into biodiesel. Environmental Chemistry Letters 16(2): 665-670.
- Santana A, Nogueira JM, Larrayoz MA (2012) Continuous production of biodiesel using supercritical fluids: A comparative study between methanol and ethanol. Fuel Processing Technology 102: 110-115.
- Taufiq-Yap YH, Lee HV, Hussein MZ, Yunus R (2011) Calcium-based mixed oxide catalysts for methanolysis of Jatropha curcas oil to biodiesel. Biomass and bioenergy 35(2): 827-834.
- Negm NA, Zahran MK, Elshafy MR, Aiad IA (2018) Transformation of Jatropha oil to biofuel using transition metal salts as heterogeneous catalysts. Journal of Molecular Liquids 256: 16-21.
- Samart C, Sreetongkittikul P, Sookman C (2009) Heterogeneous catalysis of transesterification of soybean oil using KI/mesoporous silica. Fuel Processing Technology 90(7-8): 922-925.
- Liu X, He H, Wang Y, Zhu S, Piao X (2008) Transesterification of soybean oil to biodiesel using CaO as a solid base catalyst. Fuel 87(2): 216-221.
- Tao L, Deng Y, Gan S, Chen J (2010) Application of choline chloride· xZnCl2 ionic liquids for preparation of biodiesel. Chinese Journal of Chemical Engineering 18(2): 322-327.
- Niu X, Xing C, Jiang W, Dong Y, Yuan F, et al. (2013) Activity and stability of solid base KF/La2O3 catalysts for transesterification of tributyrin with methanol. Reaction Kinetics, Mechanisms and Catalysis 109(1): 167-179.
- Forero CL (2004) Biodiesel from castor oil: A promising fuel for cold weather.
- Devaraj K, Veerasamy M, Aathika S, Mani Y, Thanarasu A et al. (2019) Study on effectiveness of activated calcium oxide in pilot plant biodiesel production. Journal of Cleaner Production 225: 18-26.
- Kubendran D, Aathika AR, Amudha T, Thiruselvi D, Yuvarani M, et al. (2017) Utilization of leather fleshing waste as a feedstock for sustainable biodiesel production. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects 39(15): 1587-1593.
- Bedir O, Dogan TH (2021) Comparison of catalytic activities of Ca-based catalysts from waste in biodiesel production. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects 1-18.
- Sakthivel R, Ramesh K, Purnachandran R, Shameer PM (2018) A review on the properties, performance and emission aspects of the third generation biodiesels. Renewable and Sustainable Energy Reviews 82: 2970-2992.
- Dugala NS, Goindi GS, Sharma A (2021) Evaluation of physicochemical characteristics of Mahua (Madhuca indica) and Jatropha (Jatropha curcas) dual biodiesel blends with diesel. Journal of King Saud University-Engineering Sciences 33(6): 424-436.
- Nakatani N, Takamori H, Takeda K, Sakugawa H (2009) Transesterification of soybean oil using combusted oyster shell waste as a catalyst. Bioresour Technol 100(3): 1510-1513.
- Syazwani ON, Rashid U, Yap YH (2015) Low-cost solid catalyst derived from waste Cyrtopleura costata (Angel Wing Shell) for biodiesel production using microalgae oil. Energy Conversion and Management 101: 749-756.
- Chen G, Shan R, Li S, Shi J (2015) A biomimetic silicification approach to synthesize CaO-SiO2 catalyst for the transesterification of palm oil into biodiesel. Fuel 153: 48-55.
- Farooq M, Ramli A, Naeem A (2015) Biodiesel production from low FFA waste cooking oil using heterogeneous catalyst derived from chicken bones. Renewable Energy 76: 362-368.
© 2023 Mayas Saad. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






