- Submissions

Full Text
Progress in Petrochemical Science
Increase Oil Recovery in Different Surfactant Concentrations on the Basis of Increasing Well Drainage Area
Eyvazov J* and Guliyeva M
Oil and Gas Research and Design Institute, SOCAR, Baku, Azerbaijan
*Corresponding author: Eyvazov J, Oil and Gas Research and Design Institute, SOCAR, Baku, Azerbaijan
Submission: December 14, 2022;Published: February 06, 2023

ISSN 2637-8035Volume5 Issue1
Abstract
Surfactant flooding is an important technology for enhanced oil recovery. A substantial amount of remaining oil resides in reservoirs, many of these are carbonate reservoirs that have low primary and water-flood recovery as a result of poor sweep efficiency that has resulted in bypassed or unswept oil. Chemical flooding methods such as surfactant flooding have been shown to be effective in recovering this unswept oil. The basis for surfactant flood is to inject a surface-active agent (a surfactant) to reduce the interfacial tension and mobilize the residual oil saturation. Surfactants have been widely used for different purposes since the early years of the petroleum industry because of their ability for altering the interfacial behavior between two immiscible fluids in contact with each other. Interfacial phenomena have an influence on rock-fluid interactions and interactions between fluids occurring from the reservoir to distribution pipelines; therefore, surfactants can be used for several petroleum industry activities. Several laboratory experiments, pilot-scale projects, and field-scale projects all over the world have shown different results regarding surfactant applications for enhancing oil recovery. Several types of surfactants have been studied to determine high-efficacy chemical EOR formulations. Anionic and non-ionic surfactants are commonly used in sandstone reservoirs.
Keywords: Enhanced oil recovery; Surfactant flooding; Waterflooding; Interfacial tension; Anionic surfactant; Non-ionic surfactant
Introduction
Surfactant flooding is an EOR technique in which surfactants and co-surfactants are injected into the reservoir to control the phase behaviour and create favourable conditions for the mobilization of stored oil. Correctly formulated surfactant solutions combined with crude oil can form micro-emulsions at the crude oil-water interface, reducing the Interfacial Tension (IFT) to very low levels (0.001mN/m), allowing residual oil to be mobilized and increased oil recovery [1]. A variety of issues, such as surfactant and co-surfactant adsorption to the rock during injection and chromatographic separation of the surfactant and cosurfactant in the reservoir, make this EOR method difficult. As a result, developing single surfactant systems would be a big step forward, as it would reduce the effects of adsorption and separation. Furthermore, the surfactants must be resistant to and active in hightemperature, high-pressure, and high-salinity reservoirs [2]. Understanding the fundamental mechanics of systems that display liquid-liquid equilibrium under reservoir conditions (e.g., oil-brine systems) is becoming increasingly important in EOR. This is true for oil and brine systems as well as sophisticated surfactant systems. Flooding, whether basic waterflooding, waterflooding where the salinity has been adjusted by the addition or removal of certain ions (so-called “smart” waterflooding), or surfactant flooding, is commonly regarded to boost oil recovery [3]. A complex chemical system is frequently pumped into the reservoir as a liquid surfactant, resulting in a micelle solution. It is critical that the complex system creates microemulsions with the leftover oil during surfactant flooding, since this aids in the reduction of the IFT and promotes mobility. Micro-emulsion production, on the other hand, may be a considerable drawback, as micro-emulsions can clog pores. It’s also crucial to be aware of the significant surfactant loss that occurs inside the reservoir due to adsorption and phase partitioning. Surfactant flooding is a method of Enhanced Oil Recovery (EOR) that increases efficiency by reducing IFT and altering wettability. Figure 1 indicates the schematic of surfactant flooding in EOR [4].
Figure 1:Schematic of a surfactant-based flooding process applied to a petroleum field.

The infusion of one or more liquid chemicals and surfactants is known as surfactant flooding. By reducing the IFT between the injected liquid and the trapped crude oil, the injection effectively modulates the phase behaviour features in the oil reservoir, mobilizing the trapped crude oil. Surfactants are used to reduce the IFT (Interfacial Tension) between oil and water. To increase the surfactant solution’s characteristics, co-surfactants are added to the liquid surfactant solution. In the blended surfactant solution, the co-surfactant acts as a promoter or an active agent to create appropriate temperature, pressure, and salinity conditions. Significant surfactant losses may occur as a result of specific physical properties of the reservoir, such as adsorption to the rock and fluid entrapment in the pore structure [5]. Surfactants and cosurfactants are commonly found in surfactant systems. However, because chromatographic separation happens in the reservoir, the mixing of several components in the surfactant solution system does not function well in practice. As the separation occurs, the concentration of the solution rapidly varies from its ideal value. The goal of surfactant flooding optimization is to increase the quantity of oil recovered while lowering the chemical expense. While it is important to achieve a low IFT for the surfactant system, minimizing only the IFT may not always correspond to optimal oil recovery, as low IFT is not the only condition to meet in order to achieve a successful and efficient oil recovery; attention to the optimal salinity is also important to include [6]. Surfactants, which are polymeric molecules that reduce the IFT between the liquid surfactant solution and the remaining oil, are surface active agents in surfactant flooding. When surfactants are present in low quantities, they adsorb on a surface or at the fluid/fluid interface. Surfactants with a nonpolar component, a hydrocarbon ‘tail’, and a polar or ionic part are the most frequent structural type. Figure 2 illustrates schematic diagram of the anionic, cationic, amphoteric, and non-ionic surfactants [7].
Figure 2:Schematic diagram of the four types of surfactants according to the composition of their heads: anionic, cationic, amphoteric, and non-ionic.
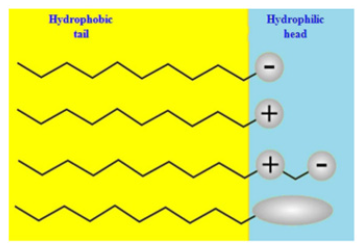
Surfactants, which are polymeric molecules that reduce the IFT between the liquid surfactant solution and the remaining oil, are surface active agents in surfactant flooding. When surfactants are present in low quantities, they adsorb on a surface or at the fluid/fluid interface. Surfactants with a nonpolar component, a hydrocarbon ‘tail,’ and a polar or ionic part are the most frequent structural type. Surfactants are typically classed as anionic, cationic, non-ionic, or zwitterionic based on the ionic nature of the head group. Depending on how the surfactant molecules ionize in aqueous liquids, each kind has distinct features Figure 3 summarizes the difference between anionic cationic and non-ionic surfactants [8]. Sulfonated hydrocarbons like alcohol propoxylate sulphate or alcohol propoxylate sulfonate are often utilized surfactants for EOR. Surfactants and polymers are frequently used in the flooding process to create the best surfactant flood for any specific oil reserve. The IFT is reduced using surfactants, and the polymer is added to increase sweep efficiency. Surfactant needs are many, and determining which pathways are the most important is difficult. In reservoir settings, process conditions like high temperature and pressure are common. Surfactants that are anionic are negatively charged. They’re often employed in detergents (alkyl benzene sulfonates), soaps (fatty acids), foaming agents (lauryl sulfate), and wetting agents, among other industrial uses (di-alkyl sulfosuccinate). In EOR, anionic surfactants are the most typically utilized. They have strong surfactant qualities, such as the capacity to reduce the IFT and generate self-assembled structures, are reasonably stable, have little adsorption on reservoir rock, and can be created cheaply. In water, anionic surfactants break down into an amphiphilic anion (negatively charged) and a cation (positively charged), which is usually an alkaline metal like sodium (Na+) or potassium (K+) [9].
Figure 3Difference between anionic cationic and nonionic surfactants.

There is no charged head group in non-ionic surfactants. They’ve also been found for usage in EOR, mostly as co-surfactants that help the surfactant process along. In aqueous solutions, their hydrophilic group is non-dissociating and does not ionize. Alcohols, phenols, ethers, esters, and amides are examples of non-ionic surfactants. Curbelo et al. investigated the relationship between non-ionic surfactants with varied degrees of ethoxylation and surfactant adsorption in porous media (sandstone). The head group of cationic surfactants is positively charged. In water, cationic surfactants dissolve into an amphiphilic cation and an anion, usually a halide (Br-,Cl-etc.). Cationic surfactants go through a high-pressure hydrogenation process during synthesis, which is often more costly than anionic surfactants. As a result, cationic surfactants are employed less frequently than anionic and nonionic surfactants [10].
The Experimental Part
In this study, Shallow Water Gunashli has been chosen for surfactant flooding. This method of EOR had done based on conventional water injection. The reservoir model was created for X horizon. There are 142 active producing wells from this horizon. Five spot water injection pattern is used in this case. All historical production, injection, pressure, perforation, and PVT data are included in the model. Firstly, history matching was done for the model. Three wells are selected for surfactant flooding purposes. They are Gun_042, Gun_081, and Gun_86. It was considered that, from July 2022, it was started to inject surfactant from these wells.
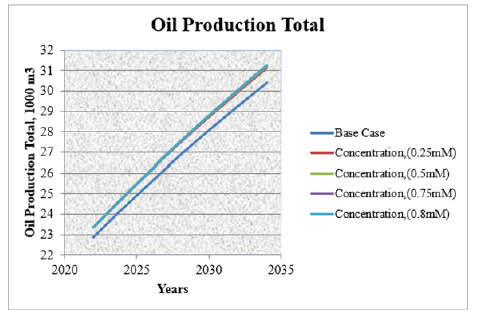
This (Table 1) represents yearly oil production rate for entire fund of producing wells of horizon X in different concentration of surfactant flooding. Oil production prediction shows that, at the end of 2034 year, yearly oil production rate will be 571.1m3 from X horizon. The highest yearly oil production rate will be in surfactant flooding with 0.75mM concentration. After 0.75mM surfactant concentration, by increasing surfactant concentration, yearly oil production rate will be same. It shows that, optimal concentration for polymer flooding in this case is 0.75mM. Figure 4 shows that, how the oil production rate will be increase as a result of surfactant flooding. In the base case, yearly oil production rate for 2034 year will be 571.1m3. But, by using surfactant with 0.75mM concentration, oil production rate will reach 590m3. After 0.75mM concentration, it will not increase oil production. Table 2 represents the increase of total oil production in different surfactant concentration in surfactant flooding in various years. Figure 5 illustrates that, how the total oil production will be increase as a result of surfactant flooding. In base case, total oil production rate in 2034 year will be 31254.852m3. But, by using surfactant with 0.75mM concentration, oil production rate will reach 30612m3. In this case, the oil recovery factor will be increased approximately 2.76%. Except that, water cut in base case was 21171.3m3 in 2034 year. But, in surfactant flooding with 0.75mM concentration, it was 21840.26m3 in 2034 year. After 0.75mM concentration, in any other increased concentration, it will not increase oil production.
Table 1:Yearly oil production rate in different concentration of surfactant flooding over the years..

Figure 4Yearly oil production rate in different concentration of surfactant flooding.
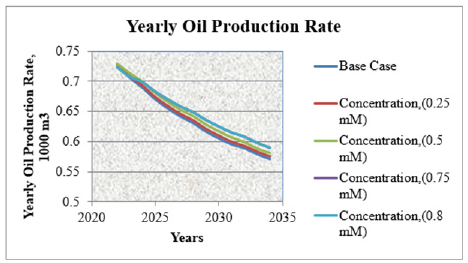
Table 2:Oil production total in different concentration of surfactant flooding over the year
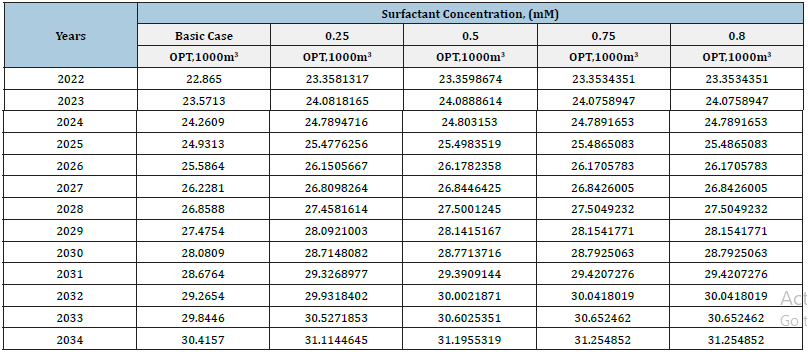
Figure /strong>Oil production total in different concentration of surfactant flooding.
Conclusion
The concentration of 0.75mM for surfactant flooding is the optimal concentration value under testing conditions because the recovery degree rose quickly, and the water content fell quickly. The degree of recovery increases as the surfactant increases. Surfactant concentration is a key aspect in the effect of surfactant flooding. After 0.75mM concentration, it doesn’t affect the oil production rate. Therefore, 0.75mM is considered optimum concentration for this case. Surfactant flooding reduces interfacial tension between the oil and water phases, also alters the wettability of the reservoir rock in order to mobilize the residual oil trapped in the reservoir, it improves volumetric sweep efficiency.
References
- Niu JG, Chen P, Shao ZB, Wang DM, Sun G, et al. (2006) Research and development of polymer enhanced oil recovery. In: Cao HQ (Ed.), Research and development of enhanced oil recovery in Daqing. Petroleum Industry Press pp. 227-325.
- Dwang DM (2001) Development of new tertiary recovery theories and technologies to sustain Daqing oil production. PGODD 20(3): 1-7.
- Sheng JJ (2013) Comparison of the effects of wettability alteration and IFT reduction on oil recovery in carbonate reservoirs. Asia-Pac J Chem Eng 8(1): 154-161.
- JSheng JJ (2010) Modern chemical enhanced oil recovery: Theory and practice, (1st edn), Elsevier, Netherlands.
- Lake lW (1989) Enhanced oil recovery. Prentice-Hall, US.
- Stegemeier GL (1977) Mechanisms of entrapment and mobilization of oil in porous media. In: Shah DO, Schechter RS (Eds.), Improved oil recovery by surfactant and polymer flooding. Academic Press, New York, US, pp. 55-91.
- Sheng JJ (2013) Review of surfactant enhanced oil recovery in carbonate reservoirs. Adv Pet Explor Dev 6(1): 1e10.
- Sheng JJ (Ed.), Surfactant enhanced oil recovery in carbonate reservoirs. Chapter 12 in EOR field case studies. Elsevier, Netherlands, pp. 281-299.
- Adibhatla B, Sun X, Mohanty KK (2005) Numerical studies of oil production from initially oil-wet fracture blocks by surfactant brine imbibitions. SPE 97687 presented at the SPE International improved oil recovery conference in Asia Pacific held in Kuala Lumpur, Malaysia.
- Delshad M, Najafabadi NF, Anderson GA, Pope GA, Sepehrnoori K (2009) Modelling wettability alteration by surfactants in naturally fractured reservoirs. SPEREE 12(3): 361-370.
© 2023 Eyvazov J. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






