- Submissions

Full Text
Progress in Petrochemical Science
Adsorptive Separation of Ethylene/Ethane by Zeolites
Chaowen Liu, Mudi Xin and Guangtong Xu*
State Key Laboratory of Catalytic Materials and Reaction Engineering, SINOPEC Research Institute of Petroleum Processing Co Ltd, China
*Corresponding author: Guangtong Xu, State Key Laboratory of Catalytic Materials and Reaction Engineering, SINOPEC Research Institute of Petroleum Processing Co Ltd, Xueyuan Road 18, Beijing, China
Submission: November 14, 2022;Published: February 02, 2023

ISSN 2637-8035Volume5 Issue1
Abstract
Adsorptive separation of ethylene/ethane based on zeolites adsorbent is a promising low-energy and high-efficiency alternative, compared with high energy-consuming cryogenic distillation procedure. In this review, the recent advances in main separation mechanisms including molecular sieving, kinetic effect, and π-complexation are surveyed and the corresponding typical zeolite sorbents are summarized. Furthermore, a perspective on possible direction to design zeolites rationally for the ethylene/ethane separation is presented.
Keywords:Separation; Zeolite; Ethylene; Ethane; Cryogenic distillation; Zeolites; Large molecules
Introduction
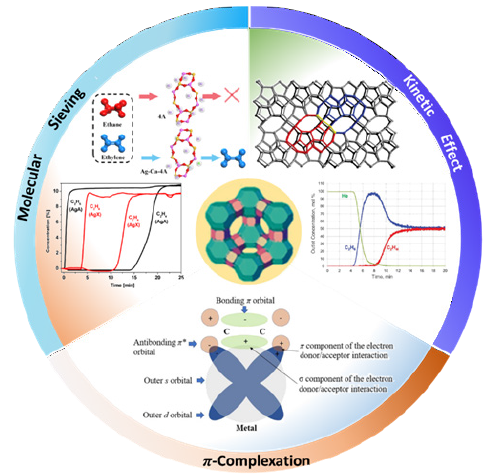
The annual demand for ethylene as a petrochemical feedstock is more than 170 million tons. Generally, ethylene is obtained from steam cracking or thermal decomposition, accompanied by ethane inevitably in the product [1]. To obtain high-purity ethylene from binary of ethylene and ethane, high-pressure cryogenic distillation is adopted due to their physical property similarities. The extremely low operating temperature and high pressure in this technique accounts for more than 70% of the energy-consuming in the entire ethylene purification process [2,3]. Therefore, for the last two decades, adsorbent-based separation technology is developed and believed gradually to be an energy-and cost-efficient alternative to replace cryogenic distillation. In adsorption process, the adsorbents are of great importance in product separation and purification performance. Zeolites were considered as ideal adsorbent than MOFs by virtue of their extraordinary stabilities, adjustable aperture size, and abundant cation adsorption active sites in industrial applications. Considering that zeolites are a promising adsorptive material in the efficient separation of ethylene and ethane, a brief review is proposed on the recent advances in adsorptive separation mechanism including molecular sieving, kinetic effect, and π-complexation (Figure 1) alongside the corresponding zeolite adsorbents, and a perspective on possible direction to design zeolites rationally for the ethylene/ethane separation is presented [4-7].
Molecular sieving
The molecular sieving enables only small and properly shaped molecules to diffuse into the zeolitic channels, whereas other large molecules are excluded, which can lead to an high selectivity. In typical ethylene/ethane separation, LTA zeolite, containing an α-cage cavity composed of six eight-membered rings (8MR), has the most appropriate aperture size of pores among the state-of-the-art structure of zeolites. And its pore size can be further regulated by tuning the quantities and types of coordination ions compensated the negative charge from the framework, such as K+, Na+, Ca2+, Mg2+, etc., varying from 3.0Å to 4.3Å. Investigations on the comparison of different metal ion-exchanged LTA zeolites for ethylene/ethane separation showed that Na-A (pore size 3.94Å) exhibited poor ethylene adsorption capacity. In addition, Ca-A (pore size 4.15Å) can achieve IAST selectivity of 4.15 (283K 100kPa) for ethylene/ethane [3]. It is noteworthy that Ca-Ag-A (pore size 4.1Å) can impede ethane completely by adjusting the orifice size to in between the molecular kinetic sizes of ethylene and ethane, thus achieving ideal molecular sieving of ethylene and ethane [4,5]. However, zeolites with suitable aperture sizes are inadequate, especially for zeolites with 8MR orifices. Even for an appropriate zeolite, there is usually a trade-off between selectivity and adsorption capacity.
Kinetic effect
Since ethylene has a smaller kinetic diameter and lower molecular weight than ethane, it has a higher rate of diffusion within the channels. And thus, ethylene/ethane separations are feasible by kinetic interaction [5]. ITQ-55 is a pure silica zeolite with a flexible structure to achieve ethylene/ethane selectivity to ~100 due to its unique pore topology with a large heart-shaped cage and framework flexibility [6]. Similarly, the gas chromatographic analysis showed that the kinetic separations of ethylene and ethane were achieved with a selectivity of up to 38 via Zn ion-exchanged ETS-4 zeolites [8]. Nevertheless, the complexity of synthesis procedure and instability of framework structure frustrates their industrial application.
π-complexation
In (Figure 1), the cations exchanged to the extra-framework of the zeolite are exceedingly prone to interact with the π-electrons of ethylene owing to its unique outer electron configuration. The outermost empty s-orbital of the metal cation and the π-molecular bonding orbitals of the ethylene forms a σ-component of the bond, while the electrons in the d-orbital of the metal cation form feedback to the vacant π* antibonding orbitals of the ethylene molecules. Based on this complexation, the zeolite was modified with Ag (I) or Cu (I) to separate ethylene and ethane. For CuCl@HY, due to the addition of Cu (I), not only the adsorption site of ethane is covered, but also the π-complexation between ethylene and active sites is established. Consequently, ethylene selectivity can reach 67 with an adsorption uptake of 2.14mmol/g [9]. Ag-A showed an excellent ethylene/ethane separation capability, and the LTA zeolite with Ag ion-exchanged achieved complete separation of ethylene and ethane with an ethylene adsorption capacity of 57cm3/g [4]. Unfortunately, the enhanced adsorptive ability generally results in difficulties in desorption, which is against the long-period operation performance.
Figure 1:Schematic diagram of the main mechanisms of ethylene/ethane separation by zeolites [4-7].
Conclusion and Outlook
A discussion of the mechanism of the separation of ethylene and ethane by summarizing the application of zeolites in the adsorption process of ethylene and ethane shows three main strategies of strengthened separation: molecular sieving, kinetic effect, and π-Complexation effect. There remains one important issue to be addressed for the design of adsorbent materials, which is the intricate balance between adsorption selectivity and capacity of the adsorbents. Combining the merits of aforementioned three mechanisms by rationally tailored pore structure and introduced active sites in zeolites can facilitate ethylene/ethane separation procedure.
References
- Li L, Lin RB, Krishna R, Li H, Xiang S, et al. (2018) Ethane/ethylene separation in a metal-organic framework with iron-peroxo sites. Science 362 (6413): 443-446.
- Sholl DS, Lively RP (2016) Seven chemical separations to change the world. Nature 532(7600): 435-437.
- Mofarahi M, Salehi SM (2012) Pure and binary adsorption isotherms of ethylene and ethane on zeolite 5A. Adsorption. 19(1): 101-110.
- Aguado S, Bergeret G, Daniel C, Farrusseng D (2012) Absolute molecular sieve separation of ethylene/ethane mixtures with silver zeolite A. J Am Chem Soc 134(36): 14635-14637.
- Anwar F, Khaleel M, Wang K, Karanikolos GN (2022) Selectivity tuning of adsorbents for ethane/ethylene separation: A Review. Industrial & Engineering Chemistry Research.
- Bereciartua PJ, Cantin A, Corma A, Jorda JL, Palomino M, et al. (2017) Control of zeolite framework flexibility and pore topology for separation of ethane and ethylene. Science 358(6366): 1068-1071.
- Liu Y, Wu Y, Liang W, Peng J, Li Z, et al. (2020) Bimetallic ions regulate pore size and chemistry of zeolites for selective adsorption of ethylene from ethane. Chem Eng Sci 220.
- Anson A, Lin CCH, Kuznicki TM, Kuznicki SM (2010) Separation of ethylene/ethane mixtures by adsorption on small-pored titanosilicate molecular sieves. Chem Eng Sci 65(2): 807-811.
- Gao F, Wang Y, Wang X Wang S (2017) Adsorptive separation of ethylene/ethane mixtures with cucl@hy adsorbent: equilibrium and reversibility. J Porous Mater 24(3): 713-719.
© 2023 Guangtong Xu. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






