- Submissions

Full Text
Progress in Petrochemical Science
Modelling and Simulation Applied to Fischer- Tropsch Synthesis
Daniel Silveira Lira*, Giovanny Silva de Oliveira, Tellys Lins Almeida Barbosa, Ciro Evandro da Silva Lobo, Fabíola Correia de Carvalho and Juan Alberto Chavez Ruiz
Sustainability Lab, Senai Institute for Innovation in Renewable Energies, Brazil
*Corresponding author: Daniel Silveira Lira, Sustainability Lab, Senai Institute for Innovation in Renewable Energies Av, Capitão-Mor Gouveia, 2770-Lagoa Nova, Natal-RN, Brazil
Submission: November 23, 2022;Published: January 09, 2023

ISSN 2637-8035Volume4 Issue5
Abstract
Fischer-Tropsch synthesis is one of the most prominent alternatives in order to substitute fossil fuels and therefore reduce GHG emissions. In order to become economically viable, the optimization of the process is necessary, and the most cost-effective way to determine its feasibility is through process modelling software. However, the behavior of this reaction is a challenge for the process simulation software due to the number of parallel and series reactions which occur simultaneously. Therefore, a guideline for the correct simulation of this process is presented in this paper
Keywords:Fischer-Tropsch synthesis; Modelling and simulation; Hydrocarbons; Sossil fuels GHG emissions
Introduction
Climate change is currently on the verge of becoming irreversible, mainly due to the high levels of Greenhouse Gas Emissions [1]. One of the major contributors to this catastrophe is the use of fossil fuels for the generation of energy, nevertheless the substitution of fossil fuels for more sustainable and clean forms of energy is currently at the utmost priority the reduce greenhouse gas emissions and keep global warming within the 1.5 °C temperature increase limit by the year of 2030 [2]. Fischer-Tropsch synthesis is one of the most promising technologies to produce fuels that can substitute fossil fuels as it can convert syngas obtained from the reform of biomasses to liquid hydrocarbons such as gasoline, diesel and SAF [3].
However, in order to become an economically feasible process, optimizations of the process are vital to determine the best operating conditions which will maximize the amount of hydrocarbons produced with desired selectivity and minimize the costs of production [4]. The optimization of a plant can become very costly if performed on the operating plant, as it consumes energy and material. Therefore, this analysis is more often performed on a process simulation software, which relies on material and energy balances to simulate the real conditions of a chemical plant. One of the main problems in using process simulation software is that some unit operations are not ideal or operate in very specific ways that cannot be simulated properly with the available generic blocks in order to obtain results that are realistic, and so, adjustments to the model must be made. This problem can be overcome by using CAPE-OPEN methodology, which permits the user to develop a custom-made model with an interface which is supported by different flowsheet applications [5]. The study of Fischer-Tropsch process using CAPE-OPEN methodology has been reported in literature. Ostadi et al. [6]. used CAPE-OPEN to study the effect of H2/CO ratio on the production and power consumption of a FT reactor, results showed that for constant CO conversion, the reactor volume could be reduced by increasing the H2/CO ratio.
Discussion
The Fischer-Tropsch process consists of a series of reactions which start with CO and H2 and behave like a series of polymerization reaction to reach hydrocarbons of bigger carbon chains, this behavior can be described by the reactions shown in equations 1 to 4, which describe the formation of methane (1), paraffins (2), olefins (3) and alcohols (4) respectively [7].

In general, the FT reaction can be processed at high temperature, also called as HTFT, which uses iron-based catalysts and is processed at temperatures between 300 and 330 °C with pressures up to 25 atm, as well as at low temperature, known as LTFT, which is operated at temperatures between 180 and 230 °C and atmospheric pressure, and uses cobalt-based catalysts [8]. Usually, the choice between LTFT and HTFT depends on the quality of syngas feedstock, as some syngas feedstocks are more prone to poison the catalyst, iron-based catalysts are preferred to be used with these kinds of feedstock, as cobalt-based catalysts are more expensive [8]. The main challenge of simulating this reaction is to include the behavior of the polymerization reactions. From literature, it is known that the mass fractions of the polymerization reactions behave as the Anderson-Schulz-Flory distribution given in equation 5 [9].

where wα is the mass fraction of the hydrocarbon with k number of carbons in its structure, and α is the chain growth probability factor, which depends on temperature and H2/CO ratio. An empirical equation for the calculation of α is given in equation 6 [9].
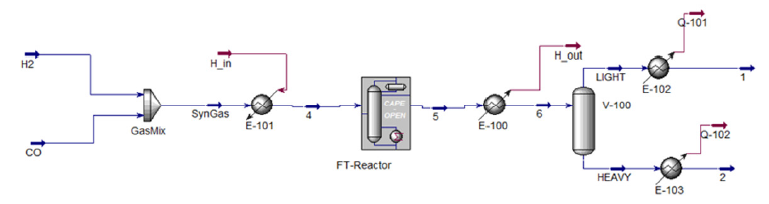
An example of a simulation flowsheet of the Fischer-Tropsch process from Aspen Hysys® is shown in (Figure 1). The flowsheet in (Figure 1) is comprised of two inlets, one containing an H2 stream and the other a CO stream. These two gases are heated up to the reaction temperature and then fed into the reactor, which in this case is a fixed bed reactor. After processing the reaction, the outlet of the reactor will be cooled down and fed into a separation drum, which separates the light hydrocarbon chains from the heavier fractions, then, both streams are cooled down to ambient temperature. Results reported in literature indicate that in order to obtain higher selectivity to C5+hydrocarbon chains, it is desirable to operate the process at 220 oC and a 2:1 H2/CO ratio. The work done by Amin et al. [10]. showed that it was possible to achieve up to 72.6% of gasoline fraction and up to 80% of diesel fraction selectivity using an iron-based catalyst with potassium promoter at these conditions. Other study by Boymans et al. [11]. It was shown that a selectivity of up to 78% C5+ could be achieved using a CO/SiO2 catalyst, also in these conditions.
Figure 1:Example of a fischer-tropsch process simulation flowsheet.
Conclusion
The authors have given the key concepts for the process modelling of the Fischer Tropsch synthesis for the production of hydrocarbons, which is one of the most prominent candidates to produce e-fuels from clean sources using green H2 ansd substitute fossil fuels, ie. gasoline, diesel and SAF.
References
- Meinshausen M, Lewis J, McGlade C, Gütschow J, Nicholls Z (2022) Realization of Paris agreement pledges may limit warming just below 2 °C. Nature 604: 304-309.
- Sadr NR, Bahrdo T, Taghizadeh R (2022) Impacts of Paris agreement, fossil fuel consumption, and net energy imports on CO2 emissions: a panel data approach for three west European Countries. Clean Technol 24(5): 1521-1534.
- Shahriar MF, Khanal A (2022 ) The current techno-economic, environmental, policy status and perspectives of sustainable aviation fuel (SAF). Fuel 325: 124905.
- Pauletto G, Galli F, Gaillardet A, Mocellin P, Patience GS (2021) Techno economic analysis of a micro gas-to-liquid unit for associated natural gas conversion. Renew Sustain Energy Rev 150: 111457.
- Ostadi M, Austbø B, Hillestad M (2019) Parametric optimization of a power and biomass to liquid process. Comput Aided Chem Eng 47: 287-292.
- Ostadi M, Rytter E, Hillestad M (2019) Boosting carbon efficiency of the biomass to liquid process with hydrogen from power: the effect of H2/CO ratio to the Fischer-Tropsch reactors on the production and power consumption. Biomass and Bioenergy 127: 105282.
- Amirov N, Vakhshouri AR (2020) Numerical modeling and optimization of product selectivity and catalyst activity in Fischer-Tropsch synthesis via response surface methodology: Cobalt carbide particle size and H2/CO ratio effects. Int J Hydrogen Energy 45(56): 31913-31925.
- Steynberg AP (2004) Chapter 1-introduction to fischer-tropsch technology. Studies in Surface Science and Catalysis Dry 152: 1-63.
- Li P, Yuan Z, Eden MR (2016) A comparative study of fischer-tropsch synthesis for liquid transportation fuels production from biomass. European Symposium on Computer Aided Process Engineering 38: 2025-2030.
- Amin M, Munir S, Iqbal N, Wabaidur SM, Iqbal A (2022) The conversion of waste biomass into carbon-supported iron catalyst for syngas to clean liquid fuel production. Catalysts 12(10): 1234.
- Boymans E, Nijbacker T, Slort D, Grootjes S, Vreugdenhil B (2022) Jet fuel synthesis from syngas using bifunctional cobalt-based catalysts. Catalysts 12(3): 288.
© 2023 Daniel Silveira Lira . This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






