- Submissions

Full Text
Progress in Petrochemical Science
Phthalocyanine Metal Macrocycles as a Nano-Catalyst for Purification of Hydrocarbon Raw Materials from Sulfur Compounds
Bassem Jamoussi*
Department of Environmental Sciences, Faculty of Meteorology, King Abdulaziz University, Saudi Arabia
*Corresponding author: Bassem Jamoussi, Department of Environmental Sciences, Faculty of Meteorology, Environment Arid Land Agriculture, King Abdulaziz University, Jeddah, Saudi Arabia
Submission: October 26, 2022;Published: December 08, 2022

ISSN 2637-8035Volume4 Issue4
Abstract
Pollution of hydrocarbon streams can be caused by hydrogen sulfide and mercaptans. Storage tanks and pipelines can also cause adverse effects on emissions. Therefore, demercaptanization and sulfur removal are ongoing research and debate topics. For the Merox® process, high pressures and temperatures are required. The development of new catalysts is of great interest. Metallophthalocyanine derivatives assist in removing crude mercaptants from hydrocarbon raw materials and increase productivity.
Keywords: Hydrocarbon stream; Demercaptanization; Metallophthalocyanines; Temperature; Raw materials
Introduction
Recently, the production of oils and gas condensates containing high levels of mercaptans, and hydrogen sulfide has increased worldwide. The chemical composition of gas condensates includes high concentrations of mercaptan sulfur, between 0.1 and 0.7% by weight, with a total sulphur content of up to 1.5% [1]. Mercaptans (C1-C3) and hydrogen sulfide (C1-C3) are toxic and volatile, and they are highly corrosive. To ensure the ecological and technological safety of storage, transportation, and processing of crude oils and gas condensates, hydrogen sulfide and low molecular weight mercaptans should be removed. An alkali metal hydroxide solution, ethyl alcohol, ketone, formaldehyde, or sodium salt of arylsulfinnic acid solution are the most common methods for mercaptan extraction from oil and gas condensates. Other methods of demercaptanization for petroleum distillates involve oxidizing mercaptans in the presence of oxygen. There are several shortcomings to these methods, including insufficient mercaptan extraction or oxidation of feed, the requirement for significant amounts of alkali or other substances, and the low stability of catalytic activity in heterogeneous catalyst systems. There is an urgent need to develop and implement catalytic desulfurization technologies that save energy and resources because of an increase in the use of sulphurous oils and gas condensates in processing, as well as a significant increase in energy consumption in the cost of production. Merox-type processes [2] are one of the best-known processes for the extraction of mercaptans and sulphur from gasoline, kerosene, and diesel fractions, with the subsequent regeneration of mercaptides with alkaline solutions. In this process using caustic solutions, light mercaptans, H2S, COS, and CS2 are removed from crude and gas condensate. Additionally, heavy mercaptans that are corrosive and active are converted into disulfides. The advantages of this process over similar methods include higher demercaptanization, higher catalyst activity stability, and more economical operation. Water-soluble metallophthalocyanines are of great scientific interest nowadays due to their of usage as efficient catalysts and photocatalysts in different oxidation processes [3,4].
Phthalocyanine Metal Macrocycles (MPc)
In recent years, scientists have become increasingly interested in metallophthalocyanines catalysts for oxidationreduction processes. An oxidative demercaptanization reaction with petroleum products would benefit from catalysts based on transition metal phthalocyanine complexes, in particular cobalt phthalocyanine complexes. Under alkaline pH conditions, Hoffmann and Lim [5] studied the auto-oxidation of hydrogen sulphide in aqueous solution catalyzed by Co(II)-, Ni(II)- and Cu(II)- tetrasulfophthalocyanine [M(II)TSP] soluble in water, the main products of the reaction of O2 with HS- in the presence of Co(II) TSP were sulfate and colloidal sulfur [5]. A major drawback of homogeneous catalysis is the separation of catalysts and oxidation products once the reaction is complete. To overcome this drawback, the water-soluble metallophthalocyanine is replaced with a hybrid/ heterogeneous complex [6,7]. In continuous-flow or batch reactors, a hybrid complex can be removed by coarse filtration, which is convenient in both situations.
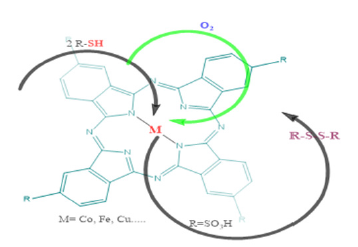
Phthalocyanine Metal Macrocycles (MPc), which possess chemical and thermal stability unique to organometallic compounds, meet most of the requirements for these catalysts. Small molecules can be reversibly bound to the active centers of such complexes and undergo redox transformations with them. Several industrial processes already use catalysts of this type (purification of hydrocarbon feedstock from sulphur compounds, oxidation of hydrocarbons). Among the phthalocyanine catalysts used in desulfurization, the most technologically advanced and convenient in operation are heterogeneous catalysts, which are produced by depositing cobalt phthalocyanine on a polymer support with salts of other metals of variable valence. Catalysts in this type provide a mass exchange between an oxygen containing gas and an oxidized product by forming effective packing elements with a geometry. Through the incorporation of phthalocyanines into polymers during polymerization using organic compounds as carriers, high stability catalysts can be produced. Ziyadova et al. [8] investigated the activity of cobalt phthalocyanine incorporated in polyamide membrane modified for the oxidation of sulfur compounds. Furthermore, the possibility of synthesizing phthalocyanine catalysts, using sol-gel chemistry has been expanding [9]. Demercaptanization and sulfur removal can be illustrated by the following scheme (Figure 1): This method has several advantages over traditional ones, including the possibility of controlling the factors that influence catalytic activity, as noted by Scott et al. [10]. During the sol-gel synthesis process, sulfonic acids formed stable bonds with silicon oxide matrix. The catalytic activity of the metal complex of phtalocyanine immobilized in the polymeric oxide matrix, exhibit pronounced catalytic activity in the R–SH type of organic compounds oxidation reaction in comparison with the individual macro-heterocycle. [9].
Figure 1:Demercaptanization and sulfur removal.
Conclusion
Several key concepts were enumerated by the author for catalytic oxidation of mercaptants in hydrocarbon raw materials. An improved hybrid/heterogeneous metllophthalocyanines complex exhibited high-performance catalytic activity for oxidizing R-SH compounds. A major advantage of heterogeneous catalysts based on phthalocyanine is their ease of removal from reaction mixtures, simplicity, and environmental friendliness.
References
- Kasperovich AT, Novopashin VF, Magaril RZ, Pestov AK (2001) Field preparation and processing of gas condensates. Tyumen, p. 80.
- Zhiyong U, Lan J, Yufei Z, Shan H, Shuangde, et al. (2014) A structured catalyst toward mercaptan sweetening with largely enhanced synergistic eff Ind Eng Chem Res 53(12): 4595-4603.
- Jamoussi B, Chakroun R, Timoumi A, Essalah K (2020) Synthesis and characterization of new imidazole phthalocyanine for photodegradation of micro-organic pollutants from sea water. Catalysts 10(8): 906.
- Vasilevich VAl, Gennadievich KA (2020) Development of catalytic systems for the purification of hydrocarbon raw materials from sulfur compounds based on phthalocyanine metal complexes. Process Management and Scientific Developments.
- Hoffmann MR, Hong APK (1987) Catalytic oxidation of reduced sulfur compounds by homogeneous and heterogeneous Co(II) phthalocyanine complexes. Science of the Total Environment 64(1-2): 99-115.
- Grubbs RH (1977) Hybrid-phase catalysts. Chemtech 7: 512-518.
- Hartley FR, Vezey PN (1977) Supported transition metal complexes as catalysts. Adv Organomet Chem 15: 189 234.
- Ziyadova TM, Burmistrov VA, Maizlish VE, Koifman OI (2017) Catalyst for the oxidation of sulfur-containing compounds based on a polyamide membrane modified with cobalt phthalocyanine. Russian Journal of Physical Chemistry A 91(3): 460-463.
- Marfin YS, Vashurin AS, Rumyantsev EV, Puhovskaya SG (2013) Sol-gel synthesis of highly effective catalyst based on cobalt tetrasulfophthalocyanine complex and silicon oxide. Journal of Sol-Gel Science and Technology 66(2): 306-311.
- Scott DW, Myers DL, Hill H, Omadoko O (2019) Sodium cobalt(II) tetrasulfophthalocyanine and catalytic oxidation of ethanethiol. Fuel 242: 573-579.
© 2022 Bassem Jamoussi. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






